Abstract
Purpose
Critically ill patients are exposed to stressful conditions and experience several discomforts. The primary objective was to assess whether a tailored multicomponent program is effective for reducing self-perceived discomfort.
Methods
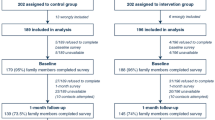
In a cluster-randomized two-arm parallel trial, 34 French adult intensive care units (ICUs) without planned interventions to reduce discomfort were randomized, 17 to the arm including a 6-month period of program implementation followed by a 6-month period without the program (experimental group), and 17 to the arm with an inversed sequence (control group). The tailored multicomponent program consisted of assessment of ICU-related self-perceived discomforts, immediate and monthly feedback to healthcare teams, and site-specific tailored interventions. The primary outcome was the overall discomfort score derived from the 16-item IPREA questionnaire (0, minimal, 100, maximal overall discomfort) and the secondary outcomes were the discomfort scores of each IPREA item. IPREA was administered on the day of ICU discharge with a considered timeframe from the ICU admission until ICU discharge.
Results
During a 1-month assessment period, 398 and 360 patients were included in the experimental group and the control group, respectively. The difference (experimental minus control) of the overall discomfort score between groups was − 7.00 (95% CI − 9.89 to − 4.11, p < 0.001). After adjustment (age, gender, ICU duration, mechanical ventilation duration, and type of admission), the program effect was still positive for the overall discomfort score (difference − 6.35, SE 1.23, p < 0.001) and for 12 out of 16 items.
Conclusions
This tailored multicomponent program decreased self-perceived discomfort in adult critically ill patients.
Trial Registration: Clinicaltrials.gov Identifier NCT02442934.



Similar content being viewed by others
References
van de Leur JP, van der Schans CP, Loef BG, Deelman BG, Geertzen JH, Zwaveling JH (2004) Discomfort and factual recollection in intensive care unit patients. Crit Care 8:R467–R473
Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, Davis SM, Morrison RS (2001) Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med 29:277–282
Novaes MA, Aronovich A, Ferraz MB, Knobel E (1997) Stressors in ICU: patients’ evaluation. Intensive Care Med 23:1282–1285
Rotondi AJ, Chelluri L, Sirio C, Mendelsohn A, Schulz R, Belle S, Im K, Donahoe M, Pinsky MR (2002) Patients’ recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med 30:746–752
Simini B (1999) Patients’ perceptions of intensive care. Lancet 354:571–572
Soehren P (1995) Stressors perceived by cardiac surgical patients in the intensive care unit. Am J Crit Care 4:71–76
Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM (2009) Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 35:796–809
Schelling G, Stoll C, Haller M, Briegel J, Manert W, Hummel T, Lenhart A, Heyduck M, Polasek J, Meier M, Preuss U, Bullinger M, Schuffel W, Peter K (1998) Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med 26:651–659
Davydow DS, Zatzick D, Hough CL, Katon WJ (2013) A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. Gen Hosp Psychiatry 35:226–232
Elliott D, McKinley S, Alison J, Aitken LM, King M, Leslie GD, Kenny P, Taylor P, Foley R, Burmeister E (2012) Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care 15:R142
Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A, Group JS (2005) Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care 9:R96–R109
Chang YJ, Pan YJ, Lin YJ, Chang YZ, Lin CH (2006) A noise-sensor light alarm reduces noise in the newborn intensive care unit. Am J Perinatol 23:265–271
Chanques G, Jaber S, Barbotte E, Violet S, Sebbane M, Perrigault PF, Mann C, Lefrant JY, Eledjam JJ (2006) Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med 34:1691–1699
Chlan LL, Weinert CR, Heiderscheit A, Tracy MF, Skaar DJ, Guttormson JL, Savik K (2013) Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: a randomized clinical trial. JAMA 309:2335–2344
Garrouste-Orgeas M, Philippart F, Timsit JF, Diaw F, Willems V, Tabah A, Bretteville G, Verdavainne A, Misset B, Carlet J (2008) Perceptions of a 24-hour visiting policy in the intensive care unit. Crit Care Med 36:30–35
Patel J, Baldwin J, Bunting P, Laha S (2014) The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia 69:540–549
Puntillo K, Arai SR, Cooper BA, Stotts NA, Nelson JE (2014) A randomized clinical trial of an intervention to relieve thirst and dry mouth in intensive care unit patients. Intensive Care Med 40:1295–1302
Flodgren G, Parmelli E, Doumit G, Gattellari M, O’Brien MA, Grimshaw J, Eccles MP (2011) Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 8:CD000125
Middleton S, McElduff P, Ward J, Grimshaw JM, Dale S, D’Este C, Drury P, Griffiths R, Cheung NW, Quinn C, Evans M, Cadilhac D, Levi C, Group QT (2011) Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet 378:1699–1706
Tainter CR, Levine AR, Quraishi SA, Butterly AD, Stahl DL, Eikermann M, Kaafarani HM, Lee J (2016) Noise Levels in Surgical ICUs Are Consistently Above Recommended Standards. Crit Care Med 44:147–152
Brandon DH, Holditch-Davis D, Belyea M (2002) Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr 140:192–199
Wessel DL (1993) Hemodynamic responses to perioperative pain and stress in infants. Crit Care Med 21:S361–S362
Boyer L, Lancon C, Baumstarck K, Parola N, Berbis J, Auquier P (2014) Evaluating the impact of a quality of life assessment with feedback to clinicians in patients with schizophrenia: randomised controlled trial. Br J Psychiatry 202:447–453
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, Selby PJ (2004) Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22:714–724
Kalfon P, Baumstarck K, Estagnasie P et al (2016) Reduction of self-perceived discomforts in critically ill patients in French intensive care units: a cluster-randomized controlled trial. Intensive Care Med Exp 4(Suppl 1):A924471
Campbell MK, Piaggio G, Elbourne DR, Altman DG, Group C (2012) Consort 2010 statement: extension to cluster randomised trials. BMJ 345:e5661
Kalfon P, Mimoz O, Loundou A, Geantot MA, Revel N, Villard I, Amour J, Azoulay E, Garrouste-Orgeas M, Martin C, Sharshar T, Baumstarck K, Auquier P (2016) Reduction of self-perceived discomforts in critically ill patients in French intensive care units: study protocol for a cluster-randomized controlled trial. Trials 17:87
Kalfon P, Mimoz O, Auquier P, Loundou A, Gauzit R, Lepape A, Laurens J, Garrigues B, Pottecher T, Malledant Y (2010) Development and validation of a questionnaire for quantitative assessment of perceived discomforts in critically ill patients. Intensive Care Med 36:1751–1758
Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE (1981) APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 9:591–597
Jackson GG, Arana-Sialer JA, Andersen Br Grieble HG, Mc CW (1962) Profiles of pyelonephritis. Arch Intern Med 110:63–75
Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O (2014) Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth 112:89–95
Kahn JM (2009) Disseminating clinical trial results in critical care. Crit Care Med 37:S147–S153
Gelinas C, Arbour C, Michaud C, Robar L, Cote J (2012) Patients and ICU nurses’ perspectives of non-pharmacological interventions for pain management. Nurs Crit Care 18:307–318
McGlothlin AE, Lewis RJ (2014) Minimal clinically important difference: defining what really matters to patients. JAMA 312:1342–1343
Acknowledgements
This research was financially supported by a grant from the Programme Hospitalier de Recherche Clinique National, 2012, 12-010-0554, funded by the French Ministry of Health. We thank all the nursing staff members and doctors whose enthusiasm and work have made this clinical trial possible. We particularly thank Anderson Loundou (Unité de recherche EA3279, Aix-Marseille Université) for participating in the statistical analysis, Bénédicte Mauchien (CH de Chartres) for her major and invaluable role in providing technical and educational support to all the investigators under the supervision of the lead investigator, and Claude Martin (CHU Hôpital Nord, AP-HM) for participating in the steering committee.
Members of the IPREA study group: Co-investigators and collaborators (alphabetically by institution, all in France) from Centre Hospitalier (CH) d’Auxerre: Karine Vie, Centre Hospitalier (CHU) de Brest: Gwenaëlle Lannuzel, CHU Beaujon, Assistance Publique-Hôpitaux de Paris (AP-HP): Hélène Bout, CHU Dijon Bourgogne: Jean-Philippe Parthiot, Isabelle Chazal, Philippe Charve, Caroline Prum, Jean-Pierre Quenot, Nora Perrot, Francis Augier, Niloufar Behechti, Claudine Cocusse, Céline Foulon, Laurence Goncalves, Abdesselem Hanchi, Etienne Legros, Ana Isabel Mercier, Nicolas Meunier-Beillard, Nathalie Nuzillat, and Alicia Richard, CH de Douai: Claire Boulle, Benjamin Kowalski, and Elisa Klusek, CHU Raymond Poincaré, AP–HP: Tarek Sharshar, Andrea Polito, Caroline Duvallet, and Sonia Krim, Groupe Hospitalier de La Rochelle-Ré-Aunis: Nicolas Girard, CH de Chartres: Juliette Audibert-Souhaid, Cécile Jourdain, and Stéphane Techer, CH Emile Roux, Le Puy-en-Velay: Corinne Chauvel, and Corinne Bruchet, CH de Lens: Johanna Temime, Stéphanie Beaussart, and Fabienne Jarosz, CHU Edouard Herriot, Hospices Civils de Lyon: Julien Crozon-Clauzel, Serge Olousouzian, Sylvie Pereira, Loïc Argentin, and Valérie Cerro, Hôpital Européen de Marseille: Déborah Levy, CHU Hôpital Nord, Assistance Publique Hôpitaux de Marseille: Sébastien Andre, Christophe Guervilly, Laurent Papazian, and Myriam Moussa, Clinique Ambroise Paré, Neuilly/Seine: Stéphanie Renoult, Delphine Biet, and Steve Novak, CHU Nice: Jean-Christophe Orban, Aminata Diop, and Carole Ichai, CHU Cochin, AP-HP: Antoine Tesniere, Jean-Pascal Goupil, and Frédérique Laville, CHU Hôpital Européen Georges Pompidou, AP-HP: Nadège Rutter, Groupe Hospitalier Paris Saint-Joseph: Sandie Brochon and Kelly Tiercelet, CHU Pitié-Salpêtrière, AP-HP: Julien Amour, Nora Ait-Hamou, and Marjorie Leger, CHU Saint-Louis, AP-HP: Virginie Souppart, CHU La Milétrie, Poitiers: Emilie Griffault, Marie-Line Debarre, Céline Deletage, Anne-Laure Guerin, Carole Guignon, and Sabrina Seguin, CH Victor Provo, Roubaix: Christophe Hart and Kathy Dernivoix, CHU Strasbourg: Caroline Wuiot, Karine Sanches, and Stéphane Hecketsweiler, Centre Hospitalier Intercommunal Toulon/La Seyne sur mer: Catherine Sylvestre-Marconville and Vincent Gardan, and CH de Troyes: Stéphanie Deparis-Dusautois and Yana Chaban.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Dr Kalfon received consulting fees from Philips Healthcare. On behalf of all remaining authors, the corresponding author states that the remaining authors have no conflicts of interest.
Additional information
Participating centers, principal investigators, members of the trial steering committee are listed in Electronic Supplementary Material (ESM)1.
Take-home message: This is the first cluster-randomized controlled study involving medical, surgical, and mixed ICUs aimed to assess the efficacy of a tailored multicomponent program based on a simultaneous approach to reduce discomfort from multiple sources, by combining assessment of self-perceived discomfort, feedback to the healthcare teams, and tailored site-targeted measures under control of a duo of local champions. Our intervention, easily reproducible in different settings, reduced perceived overall discomfort in unselected adult critically ill patients. Based on this positive result, the study paves the way for a new strategy for care management in the ICU.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalfon, P., Baumstarck, K., Estagnasie, P. et al. A tailored multicomponent program to reduce discomfort in critically ill patients: a cluster-randomized controlled trial. Intensive Care Med 43, 1829–1840 (2017). https://doi.org/10.1007/s00134-017-4991-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4991-x