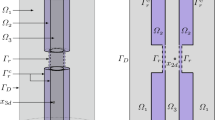
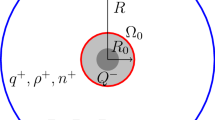
Abstract
We report here new electrical laws, derived from nonlinear electrodiffusion theory, about the effect of the local geometrical structure, such as curvature, on the electrical properties of a cell. We adopt the Poisson–Nernst–Planck equations for charge concentration and electric potential as a model of electrodiffusion. In the case at hand, the entire boundary is impermeable to ions and the electric field satisfies the compatibility condition of Poisson’s equation. We construct an asymptotic approximation for certain singular limits to the steady-state solution in a ball with an attached cusp-shaped funnel on its surface. As the number of charge increases, they concentrate at the end of cusp-shaped funnel. These results can be used in the design of nanopipettes and help to understand the local voltage changes inside dendrites and axons with heterogeneous local geometry.







Similar content being viewed by others
References
Bezanilla, F.: How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9, 323–332 (2008)
Cartailler, J., Schuss, Z., Holcman, D.: Analysis of the Poisson-Nernst-Planck equation in a ball for modeling the voltage-current relation in neurobiological microdomains. Phys. D Nonlinear Phenom. 339, 39–48 (2016)
Courant, R., Hilbert, D.: Methods of Mathematical Physics, vol. 2. Wiley Interscience, New York (1989)
Debye, P., Hückel, E.: Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen. Phys. Z. 24(9), 185–206 (1923)
Delgado, M., Coombs, D.: Conditional mean first passage times to small traps in a 3-D domain with a sticky boundary. SIAM J. Multiscale Anal. Simul. 13(4), 1224–1258 (2015)
Eisenberg, R.S.: From structure to function in open ionic channels. J. Membr. Biol. 171, 1–24 (1998a)
Eisenberg, R.S.: Ionic channels in biological membranes. Electrostatic analysis of a natural nanotube. Contemp. Phys. 39(6), 447–466 (1998b)
Eisenberg, R.S., Klosek, M.M., Schuss, Z.: Diffusion as a chemical reaction: stochastic trajectories between fixed concentrations. J. Chem. Phys. 102(4), 1767–1780 (1995)
Hille, B.: Ion Channels of Excitable Membranes, 3rd edn. Sinauer Associates, Sunderland (2001)
Holcman, D., Schuss, Z.: Brownian motion in dire straits. SIAM J. Multiscale Model. Simul. 10(4), 1204–1231 (2012a)
Holcman, D., Schuss, Z.: Brownian motion in dire straits. Multiscale Model. Simul. 10(4), 1204–1231 (2012b)
Holcman, D., Schuss, Z.: Stochastic Narrow Escape in Molecular an Cellular Biology. Analysis and Applications. Springer, New York (2015)
Holcman, D., Yuste, R.: The new nanophysiology: regulation of ionic flow in neuronal subcompartments. Nat. Rev. Neurosci. 16, 685–692 (2015)
Holcman, D., Hoze, N., Schuss, Z.: Narrow escape through a funnel and effective diffusion on a crowded membrane. Phys. Rev. E 84, 021906 (2011). Erratum. Phys. Rev. E 85, 039903 (2012)
Horn, R., Roux, B., Aqvist, J.: Permeation redux: thermodynamics and kinetics of ion movement through potassium channels. Biophys J. 106(9), 1859–1863 (2014)
Lindsay, A.E., Bernoff, A.J., Ward, M.J.: First passage statistics for the capture of a Brownian particle by a structured spherical target with multiple surface traps. SIAM J. Multiscale Model. Simul. 15(1), 74–109 (2016)
Mamonov, A., Coalson, R., Nitzan, A., Kurnikova, M.: The role of the dielectric barrier in narrow biological channels: a novel composite approach to modeling single channel currents. Biophys. J. 84, 3646–3661 (2003)
Perry, D., Momotenko, D., Lazenby, R.A., Kang, M., Unwin, P.R.: Characterization of nanopipettes. Anal. Chem. 88(10), 5523–5530 (2016)
Pillay, S., Peirce, A., Kolokolnikov, T., Ward, M.: An asymptotic analysis of the mean first passage time for narrow escape problems: part I: two-dimensional domains. SIAM Multiscale Model. Simul. 8(3), 803–835 (2010)
Qian, N., Sejnowski, T.J.: An electro-diffusion model for computing membrane potentials and ionic concentrations in branching dendrites, spines and axons. Biol. Cybern. 62, 1–15 (1989)
Rall, W.: Cable theory for dendritic neurons. In: Koch, C., Segev, I. (eds.) Methods in Neuronal Modeling: From Synapses to Networks, pp. 9–63. MIT Press, Cambridge (1989)
Roux, B., Karplus, M.: Ion transport in the gramicidin channel: free energy of the solvated right-handed dimer in a model membrane. J. Am. Chem. Soc. 115, 3250–3262 (1993)
Ruiz, F.J.G., Godoy, A., Gamiz, F., Sampedro, C., Donetti, L.: A comprehensive study of the corner effects in Pi-gate MOSFETs including quantum effects. IEEE Trans. Electron Devices 54(12), 3369–3377 (2007)
Savtchenko, L.P., Kulahin, N., Korogod, S.M., Rusakov, D.A.: Electric fields of synaptic currents could influence diffusion of charged neurotransmitter molecules. Synapse 51(4), 270–278 (2004)
Schuss, Z., Nadler, B., Eisenberg, R.S.: Derivation of Poisson and Nernst-Planck equations in a bath and channel from a molecular model. Phys. Rev. E 64, 036116 (2001)
Singer, A., Norbury, J.: A Poisson-Nernst-Planck model for biological ion channels–an asymptotic analysis in a three-dimensional narrow funnel. SIAM Appl. Math. 7(3), 949–968 (2009)
Sparreboom, W., van den Berg, A., Eijkel, J.C.T.: Principles and applications of nanofluidic transport. Nat. Nanotechnol 4, 713–720 (2009)
Yuste, R.: Dendritic Spines. The MIT Press, Cambridge (2010)
Acknowledgements
Funding was provided by H2020 Marie Skłodowska-Curie Actions (Grant No. 4328435).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Philip K. Maini.
Appendix
Appendix
1.1 Regular Expansion of the PNP Solution when There are an Excess of Positive and a Small Number of Negative Charges
We show that for the concentrations of cations and anions found in the literature (Hille 2001), the leading-order solution of the electrical potential in the bulk can be obtained by considering positive charges only. We assume that the charge of an electrolyte confined in \(\tilde{\varOmega }\) consists of identical \(N_{p}\) positive and \(N_m\) negative ions with density \(q_{p}({\varvec{x}})\) and \(q_m({\varvec{x}})\) such as
where p and m are positive and negative species, respectively. The total charges in \(\tilde{\varOmega }\) are the sum
The associated charge densities \(\rho _{p}({\varvec{x}},t)\) and \(\rho _m({\varvec{x}},t)\) satisfy the boundary value problem for the Nernst–Planck equation
where \(z_i\) is the valence and \(D_i\) is the diffusion coefficient for the ion specie i. The electric potential \(\phi ({\tilde{{\varvec{x}}}},t)\) in \(\tilde{\varOmega }\) is solution of the Neumann problem for the Poisson equation
where \(\tilde{\sigma }({\tilde{{\varvec{x}}}},t)\) is the surface charge density on the boundary \(\partial \tilde{\varOmega }\). At steady state, (119) gives
where \(\rho _{i,0} \) is obtained from no-flux boundary condition (120), thus
Using the nondimensionalized potential \(\displaystyle \tilde{u}({\tilde{{\varvec{x}}}})=\frac{e\,\phi ({\tilde{{\varvec{x}}}})}{k_\mathrm{BT}}\), Eq. (123) becomes
Using (122) and (125), we obtain
where \(l_\mathrm{B}\) is the Bjerrum length. Using \(\displaystyle {\varvec{x}}=\frac{{\tilde{{\varvec{x}}}}}{R_\mathrm{c}}\) and \(\tilde{u}(\tilde{x})=u(x)\) where \(R_\mathrm{c}\) is the cusp curvature radius, (126) becomes
The small parameter is \(\zeta =\displaystyle \frac{N_m}{N_p}\ll 1\) because in the bulk, the concentration of negative charges such as chloride (about 4 mM) is much smaller than positive ones (potassium and sodium account together roughly for 167 mM Hille 2001). A regular expansion of \(u({\varvec{x}})\) is
Using (128) in (127), in small \(\zeta \) limit, we have
and in \(\varOmega \)
which admit a regular solution. This result shows that the limit of the PNP equation when \(\zeta \) tends to zero (small charge limit) gives \(v_0({\varvec{x}})\), and thus we conclude that a small amount of negative charges does not perturb the distribution of the total excess of positive charge in the bulk.
1.2 The Numerical Procedure
Numerical solutions were constructed by the COMSOL Multiphysics 5.0 (BVP problems), Maple 2015 (shooting problems) and MATLAB R2015 (conformal mapping). The boundary value problems in 1D, 2D and 3D were solved by the finite elements method in the COMSOL ‘Mathematics’ package. We used an adaptive mesh refinement to ensure numerical convergence for large value of the parameter \(\lambda \).
We solved the PDEs by the shooting procedure for boundary value problems using Runge–Kutta fourth-order method, as well as solvers from Maple packages.
Rights and permissions
About this article
Cite this article
Cartailler, J., Schuss, Z. & Holcman, D. Geometrical Effects on Nonlinear Electrodiffusion in Cell Physiology. J Nonlinear Sci 27, 1971–2000 (2017). https://doi.org/10.1007/s00332-017-9393-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00332-017-9393-2
Keywords
- Electro-diffusion
- Poisson–Nernst–Planck
- Electrolytes
- Neurobiology
- Asymptotics
- Mobius conformal map
- Nonlinear partial differential equation
- Curvature
- Cusp–shaped funnel
- Nonelectroneutrality