Abstract
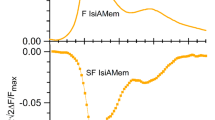
Five variants of glucokinase (ATP-D-hexose-6-phosphotransferase, EC 2.7.1.1) including wild type and single Trp mutants with the Trp residue at positions 65, 99, 167 and 257 were prepared. The fluorescence of Trp in all locations studied showed intensity changes when glucose bound, indicating that conformational change occurs globally over the entire protein. While the fluorescence quantum yield changes upon glucose binding, the enzyme’s absorption spectra, emission spectra and fluorescence lifetimes change very little. These results are consistent with the existence of a dark complex for excited state Trp. Addition of glycerol, L-glucose, sucrose, or trehalose increases the binding affinity of glucose to the enzyme and increases fluorescence intensity. The effect of these osmolytes is thought to shift the protein conformation to a condensed, high affinity form. Based upon these results, we consider the nature of quenching of the Trp excited state. Amide groups are known to quench indole fluorescence and amides of the polypeptide chain make interact with excited state Trp in the relatively unstructured, glucose-free enzyme. Also, removal of water around the aromatic ring by addition of glucose substrate or osmolyte may reduce the quenching.










Similar content being viewed by others
References
Grossbard L, Schimke RT (1968) Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem 241:3546–3560
Matschinsky FM, Ellerman JE (1968) Metabolism of glucose in the islets of Langerhans. J Biol Chem 243:2730–2736
Schimke RT, Grossbard L (1968) Studies isozymes of hexokinase in animal tissues. Ann N Y Acad Sci 151:332–350
Matschinsky FM (1990) Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 39:647–652
Matschinsky FM (2005) Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep 5:171–176
Van Schaftingen E, Detheux M, Veiga de Cunha M (1994) Short-term control of glucokinase activity: role of a regulatory protein. FASEB J 8:414–419
Osbak KK, Colclough K, Saint-Martin, C, Beer NL, Bellanne-Chantelot C, Ellard S, Gloyn AL (2009) Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes and hyperinsulinemic hypoglycemis. Hum Mutat 30:1512–1526
Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF (2003) Allosteric activators of glucokinase: potential role in diabetes. Science 301:370–373
Matschinsky FM (2013) GKAs for diabetes therapy: why no clinically used drugs after two decades of trying? Trends Pharmacol Sci 34:90–99
Kamata K, Mitsuya M, Nishimura T, Eiki Jun-ichi, Nagata Y (2004) Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 12:429–438
Larion M, Salinas RK, Bruschweiler-Li L, Bruschweiler R, Miller BG (2010) Direct evidence of conformational heterogeneity in human pancreatic glucokinase from high-resolution nuclear magnetic resonance. Biochemistry 49:7969–7971
Zelent B, Buettger C, Grimsby J, Sarabu R, Vanderkooi JM, Wand AJ, Matschinsky FM (2012) Thermal stability of glucokinase (GK) as influenced by the substrate glucose, and allosteric glucokinase activator drug (GKA) and the osmolytes glycerol and urea. Biochim Biophys Acta 1824:769–784
Zelent B, Raimondo A, Barrett A, Buettger CW, Chen P, Gloyn AL, Matschinsky FM (2014) Analysis of the co-operative interaction between the allosterically regulated proteins GK and GKRP using tryptophan fluorescence. Biochem J 459:551–564
Zelent B, Odili S, Buettger C, Shiota C, Grimsby J, Taub R, Magnuson MA, Vanderkooi JM, Matschinsky FM (2008) Sugar binding to recombinant wild-type and mutant glucokinase monitored by kinetic measurement and tryptophan fluorescence. Biochem J 413:269–280
Zelent B, Odili S, Buettger C, Zelent DK, Chen P, Fenner D, Bass J, Stanley C, Laberge M, Vanderkooi JM, Sarabu R, Grimsby J, Matschinsky FM (2011) Mutational analysis of allosteric activation and inhibition of glucokinase. Biochem J 440:203–215
Chen RF, Knutson JR, Ziffer H, Porter D (1991) Fluorescence of tryptophan dipeptides: correlations with the rotamer model. Biochemistry 30:5184–5195
Xu J, Knutson JR (2009) Quasi-static self-quenching of Trp-X and X-Trp dipeptides in water: ultrafast fluorescence decay. J Phys Chem B 113:12084–12089
Vagenende V, Yap MG, Trout BL (2009) Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 48:11084–11096
Liang Y, Kesavan P, Wang LQ, Niswender K, Tanizawa, Y, Permutt, MA, Magnuson MA, Matschinsky FM (1995) Variable effects of maturity-onset-diabetes-of-youth (MODY)-associated glucokinase mutations on substrate interactions and stability of the enzyme. Biochem J 309:167–173
Davis EA, Cuesta-Munoz A, Raoul M, Buettger C, Sweet I, Moates M, Magnuson MA, Matschinsky FM (1999) Mutants of glucokinase cause hypoglycaemia and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 42:1176–1186
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, New York
Szabo AG, Rayner DM (1980) Fluorescence decay of tryptophan conformers in aqueous solutions. J Am Chem Soc 102:554–563
Konev SV (1967) Fluorescence and phosphorescence of proteins and nucleic acids. Penum Press, New York
Dashnau JL, Sharp KA, Vanderkooi JM (2005) Stereochemical aspects of aldohexopyranose hydration as studied by water-water hydrogen bond angle analysis. J Phys Chem 109:24152–24159
Zelent B, Vanderkooi JM (2009) Infrared spectroscopy used to study ice formation: the effect of trehalose, maltose and glucose on melting. Anal Biochem 390:215–217
Alcala JR, Gratton E, Prendergast FG (1987) Fluorescence lifetime distributions in proteins. Biophys J 51:597–604
Toptygin D, Brand L (2000) Spectrally- and time-resolved fluorescence emission of indole during solvent relaxation: a quantitative model. Chem Phys Lett 322:496–502
Niemyer H, de la Luz Cardenase M, Rabajille E, Ureta T, Clark-Turri L, Penaranda J (1975) Sigmoidal kinetics of glucokinase. Enzyme 20:321–333
Storer AC, Cornish-Bowden A (1976) Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem J 159:7–14
Liu S, Ammirati MJ, Song X, Knafeis JD, Zhang J, Greasley SE, Pfefferkorn JA, Qui X (2012) Insights into mechanism of glucokinase activation: observation of multiple distinct protein conformations. J Biol Chem 287:13598–13610
Kim YB, Kalinowski SS, Marcinkeviciene J (2007) A pre-steady state analysis of ligand binding to human glucokinase: evidence for a preexisting equilibrium. Biochemistry 46:1423–1431
Chen Y, Barkley MD (1998) Toward understanding tryptophan fluorescence in proteins. Biochemistry 37:9976–9982
Callis PR, Vivian JT (2003) Understanding the variable fluorescence quantum yield of tryptophan in proteins using QM-MM simulations. Quenching by charge transfer to the peptide backbone. Chem Phys Lett 369:409–414
Albani JR (2014) Origin of tryptophan fluorescence lifetimes. Part 2: fluorescence lifetimes origin of trytophan in proteins. J Fluoresc 24:105–117
Gekko K, Timasheff SN (1981) Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry 20:4667–4676
Pickup JC, Hussain R, Evans BD, Rolinski OJ, Birch DJS (2005) Fluorescence-based glucose sensors. Biosens Bioelectron 20:2555–2565
Acknowledgements
The work was supported by grants from NIDDK no. 22122 and 19,525 (F.M.M.), NSF grant CBET-1264608 (I.G.) and National Research Initiative or Agriculture and Food Research Initiative Grant no. 2014–67,017-2149 (R.D.L., B.Z.& M.G.C.) from USDA National Institute of Food and Agriculture, Improving Food Quality.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zelent, B., Bialas, C., Gryczynski, I. et al. Tryptophan Fluorescence Yields and Lifetimes as a Probe of Conformational Changes in Human Glucokinase. J Fluoresc 27, 1621–1631 (2017). https://doi.org/10.1007/s10895-017-2099-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2099-x