Abstract
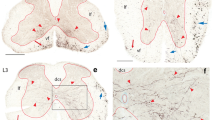
The A11 dopaminergic cell group is the only group among the A8–A16 dopaminergic cell groups that includes neurons innervating the spinal cord, and a decrease in dopaminergic transmission at the spinal cord is thought to contribute to the pathogenesis of restless legs syndrome. However, the mechanisms regulating the neuronal activity of A11 dopaminergic neurons remain to be elucidated. Unraveling the neuronal composition, distribution and connectivity of A11 neurons would provide insights into the mechanisms regulating the spinal dopaminergic system. To address this, we performed immunohistochemistry for calcium-binding proteins such as calbindin (Calb) and parvalbumin (PV), in combination with the retrograde tracer Fluorogold (FG) injected into the spinal cord. Immunohistochemistry for Calb, PV, or tyrosine hydroxylase (TH), a marker for dopaminergic neurons, revealed that there were at least three types of neurons in the A11 region: neurons expressing Calb, TH, or both TH and Calb, whereas there were no PV-immunoreactive (IR) cell bodies. Both Calb- and PV-IR processes were found throughout the entire A11 region, extending in varied directions depending on the level relative to bregma. We found retrogradely labeled FG-positive neurons expressing TH, Calb, or both TH and Calb, as well as FG-positive neurons lacking both TH and Calb. These findings indicate that the A11 region is composed of a variety of neurons that are distinct in their neurochemical properties, and suggest that the diencephalospinal dopamine system may be regulated at the A11region by both Calb-IR and PV-IR processes, and at the terminal region of the spinal cord by Calb-IR processes derived from the A11 region.





Similar content being viewed by others
References
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30(5):194–202
Skagerberg G, Lindvall O (1985) Organization of diencephalic dopamine neurons projecting to the spinal cord in the rat. Brain Res 342(2):340–351
Thorpe AJ, Clair A, Hochman S, Clemens S (2011) Possible sites of therapeutic action in restless legs syndrome: focus on dopamine and α2δ ligands. Eur Neurol 66(1):18–29
Charbit AR, Akerman S, Holland PR, Goadsby PJ (2009) Neurons of the dopaminergic/calcitonin gene-related peptide A11 cell group modulate neuronal firing in the trigeminocervical complex: an electrophysiological and immunohistochemical study. J Neurosci 29(40):12532–12541
Koblinger K, Füzesi T, Ejdrygiewicz J, Krajacic A, Bains JS, Whelan PJ (2014) Characterization of A11 neurons projecting to the spinal cord of mice. PLoS ONE 9(10):e109636
van den Pol AN (1986) Tyrosine hydroxylase immunoreactive neurons throughout the hypothalamus receive glutamate decarboxylase immunoreactive synapses: a double pre-embedding immunocytochemical study with particulate silver and HRP. J Neurosci 6(3):877–891
Pappas SS, Tiernan CT, Behrouz B, Jordan CL, Breedlove SM, Goudreau JL et al (2010) Neonatal androgen-dependent sex differences in lumbar spinal cord dopamine concentrations and the number of A11 diencephalospinal dopamine neurons. J Comp Neurol 518(13):2423–2436
Abdallah K, Monconduit L, Artola A, Luccarini P, Dallel R (2015) GABAAergic inhibition or dopamine denervation of the A11 hypothalamic nucleus induces trigeminal analgesia. Pain 156(4):644–655
Rogers JH (1992) Immunohistochemical markers in rat brain: colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain Res 587(2):203–210
González-Hernández T, Rodríguez M (2000) Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421(1):107–135
Olson VG, Nestler EJ (2007) Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse 61(2):87–95
Arai R, Jacobowitz DM, Deura S (1994) Distribution of calretinin, calbindin-D28k, and parvalbumin in the rat thalamus. Brain Res Bull 33(5):595–614
Gerfen CR, Baimbridge KG, Thibault J (1987) The neostriatal mosaic: III. Biochemical and developmental dissociation of patch-matrix mesostriatal systems. J Neurosci 7(12):3935–3944
Gerfen CR, Herkenham M, Thibault J (1987) The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci 7(12):3915–3934
Celio MR (1990) Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35(2):375–475
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press/Elsevier, Boston.
Carr DB, Sesack SR (2000) GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse 38(2):114–123
Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK (2012) The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cereb Cortex 22(2):327–336
Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H et al (2006) Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol 498(5):581–592
Li X, Qi J, Yamaguchi T, Wang HL, Morales M (2013) Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct 218 (5):1159–1176
Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152(4):1024–1031
Tsumori T, Qin Y, Yokota S, Niu JG, Yasui Y (2010) Central amygdaloid axon terminals are in contact with retrorubral field neurons that project to the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res 1306:18–28
Yamaguchi T, Sheen W, Morales M (2007) Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci 25(1):106–118
Yamaguchi T, Wang HL, Li X, Ng TH, Morales M (2011) Mesocorticolimbic glutamatergic pathway. J Neurosci 31(23):8476–8490
Yamaguchi T, Wang HL, Morales M (2013) Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur J Neurosci 38(11):3602–3610
Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci 41(6):760–772
Neuhoff H, Neu A, Liss B, Roeper J (2002) I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci 22(4):1290–1302
Wang HL, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29(2):340–358
Martinez-Gonzalez C, Wang HL, Micklem BR, Bolam JP, Mena-Segovia J (2012) Subpopulations of cholinergic, GABAergic and glutamatergic neurons in the pedunculopontine nucleus contain calcium-binding proteins and are heterogeneously distributed. Eur J Neurosci 35(5):723–734
Frassoni C, Spreafico R, Bentivoglio M (1997) Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res 115(1):95–104
Hu H, Gan J, Jonas P (2014) Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345(6196):1255263
Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP et al (1987) Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 66(1):191–210
Moriizumi T, Hattori T (1992) Anatomical and functional compartmentalization of the subparafascicular thalamic nucleus in the rat. Exp Brain Res 90(1):175–179
Pappas SS, Kennedy T, Goudreau JL, Lookingland KJ (2011) Opioid-mediated regulation of A11 diencephalospinal dopamine neurons: pharmacological evidence of activation by morphine. Neuropharmacol 61 (4):614–621.
Acknowledgements
This work was supported by Dokkyo Medical University. H.O. is the recipient of a Young Investigator award (No. 2015-21) from Dokkyo Medical University. We would like to thank Shukuko Nihei for excellent technical assistance.
Funding
This study was funded by a Young Investigator Award (Grant No. 2015-21 for H.O.) from Dokkyo Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Hidechika Ozawa and Tsuyoshi Yamaguchi have contributed equally to do this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ozawa, H., Yamaguchi, T., Hamaguchi, S. et al. Three Types of A11 Neurons Project to the Rat Spinal Cord. Neurochem Res 42, 2142–2153 (2017). https://doi.org/10.1007/s11064-017-2219-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2219-7