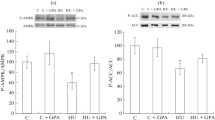
The effects of different durations of gravitational unloading (antiorthostatic suspension of rats for 1, 3, or 7 days) on the intensity protein synthesis, ribosomal RNA contents, and intracellular signal pathways involved in controlling protein biosynthesis were studied in rat soleus muscle. The contents of key markers of the anabolic signal pathways (p-AKT, p-GSK-3β, p-p70s6k, p-4E-BP1, p-90RSK1) were determined by gel electrophoresis followed by immunoblotting. The intensity of protein synthesis in soleus muscle was evaluated by puromycin labeling, i.e., the SUnSET method. A significant decrease in protein synthesis intensity in the soleus muscle was seen after 3 and 7 days of gravitational unloading. Gravitational unloading for 3 and 7 days led to a significant decrease in the content of 28S rRNA. Functional unloading for 24 h led to a significant increase in the content of p-p70s6k and a decrease in the content of p-4E-BP1 in the soleus muscle (p < 0.05). By day 3 of suspension, there were decreases in the contents of markers such as phospho-AKT and phospho-p90RSK1 as compared with controls (p < 0.05). After 7 days of gravitational unloading, there were significant decreases in the levels of phosphorylated AKT and GSK-3β compared with the control group (p < 0.05). These results lead to the conclusion that decreases in the intensity of protein synthesis in rat soleus muscle at the early stages of gravitational unloading may be associated with suppression of ribosome biogenesis and decreases in the activity of mTORC1-dependent signal pathways.
Similar content being viewed by others
References
E. A. Lysenko, O. V. Turtikova, E. V. Kachaeva, et al., “Activity of ribosomal kinases in functional unloading of different durations,” Dokl. Akad. Nauk., 434, No. 1, 126–129 (2010).
A. M. Krasnyi, E. A. Lysenko, I. B. Kozlovskaya, et al., “Phosphorylation of elongation factor and expression of its kinase in rat soleus muscle during three days of gravitational unloading,” Dokl. Akad. Nauk., 453, No. 1, 1–3 (2013).
G. Bajotto, Y. Sato, Y. Kitaura, and Y. Shimomura, “Effect of branchedchain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles,” Eur. J. Appl. Physiol., 111, No. 8, 1815–1828 (2011).
S. C. Bodine, “Disuse-induced muscle wasting,” Int. J. Biochem. Cell Biol., 45, 2200–2208 (2013).
J. Cannavino, L. Brocca, M. Sandri, et al., “PGC 1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice,” J. Physiol., 592, No. 20, 4575–4589 (2014).
T. Chaillou, T. J. Kirby, and J. J. McCarthy, “Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass,” J. Cell Physiol., 229, 1584–1594 (2014).
E. Dupont, C. Cieniewski-Bernard, B. Bastide, and L. Stevens, “Electrostimulation during hindlimb unloading modulates PI3KAKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 300, 408–417 (2001).
R. H. Fitts, D. R. Wiley, and J. Widrick, “Physiology of a microgravity environment invited review: microgravity and skeletal muscle,” J. Appl. Physiol., 89, 823–839 (2000).
J. D. Fluckey, E. E. Dupont-Versteegden, D. C. Montague, et al., “A rat resistance exercise regimen attenuates fosses of musculoskeletal mass during hindlimb suspension,” Acta Physiol. Scand., 176, No. 4, 293–300 (2002).
J. Fluckey, E. Dupont-Versteegden, M. Knox, et al., “Insulin facilitation of muscle protein synthesis following resistance exercise in hindlimb-suspended rats is independent of a rapamycin sensitive pathway,” Am. J. Physiol. Endocrinol. Metab., 287, 1070–1075 (2004).
D. J. Glass, “Signalling pathways that mediate skeletal muscle hypertrophy and atrophy,” Nat. Cell, Biol., 5, 87–90 (2003).
C. Goodman, D. Mabrey, J. Frey, et al., “Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique,” FASEB J., 25, No. 3, 1028–1039 (2011).
T. Gwag, K. Lee, H. Ju, et al., “Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: a catch-up process for atrophied muscle,” Cell Physiol. Biochem., 24, No. 5–6, 537–546 (2009).
T. Hornberger, R. Hunter, S. Kandarian, and K. Esser, “Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats,” Am. J. Physiol. Cell Physiol., 281, 179–187 (2001).
T. A. Hornberger and S. Chien, “Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle,” J. Cell. Biochem., 97, 1207–1216 (2006).
S. R. Kimball and L. S. Jefferson, “Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise,” J. Biol. Chem., 285, 29,027–29,032 (2010).
U. K. Laemmli, “Cleavage of structural proteins during the assembly of the head of bacteriophage T4,” Nature, 227, No. 5259, 680–685 (1970).
P. Loughna, G. Goldspink, and D. F. Goldspink, “Effect of inactivity and passive stretch on protein turnover in phasic and postural rat muscles,” J. Appl. Physiol., 61, No. 1, 173–179 (1986).
A. Matsakas and K. Patel, “Intracellular signalling pathways regulating the adaptation of skeletal muscle to exercise and nutritional changes,” Histol. Histopathol., 24, No. 2, 209–222 (2009).
J. J. McCarthy and K. A. Esser, “Anabolic and catabolic pathways regulating skeletal muscle mass,” Curr. Opin. Clin. Nutr. Metab. Care, 13, No. 3, 230–235 (2010).
E. Morey-Holton and R. Globus, “Hindlimb unloading rodent model: technical aspects,” J. Appl. Physiol., 92, 1367–1377 (2002).
D. Nathans, “Puromycin inhibition of protein synthesis: incorporation of puromycin into peptide chains,” Proc. Natl. Acad. Sci. USA, 51, 585–592 (1964).
S. Phillips and C. McGlory, “CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis,” J. Physiol., 592, 5341–5343 (2014).
M. Reid, R. Judge, and S. Bodine, “CrossTalk opposing view: The dominant mechanism causing disuse muscle atrophy is proteolysis,” J. Physiol., 592, 5345–5347 (2014).
M. J. Rennie, A. Selby, P. Atherton, et al., “Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy,” Scand. J. Med. Sci. Sports, 20, 5–9 (2010).
P. P. Roux and I. Topisirovic, “Regulation of mRNA translation by signaling pathways,” Cold Spring Harb. Perspect. Biol., 4, a012252 (2012).
E. K. Schmidt, “SUnSET, a nonradioactive method to monitor protein synthesis,” Nat. Methods, 6, No. 4, 275–277 (2009).
T. Sugiura, N. Abe, M. Nagano, et al., “Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 288, No. 5, 1273–1278 (2005).
J. L. Van der Velden, R. C. Langen, M. C. Kelders, et al., “Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta,” Am. J. Physiol. Cell Physiol., 292, No. 5, 1636–1644 (2007).
N. A. Vilchinskaya, T. M. Mirzoev, Y. N. Lomonosova, et al., “Human muscle signaling responses to 3-day head-out dry immersion,” J. Musculoskel. Neuron Interact., 15, No. 3, 286–293 (2015).
J. S. You, G. B. Anderson, M. S. Dooley, and T. A. Hornberger, “The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization,” Dis. Model Mech., pii: dmm. 019414. Epub (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 101, No. 11, pp. 1299–1308, November, 2015.
Rights and permissions
About this article
Cite this article
Mirzoev, T.M., Tyganov, S.A., Lomonosova, Y.N. et al. Signaling Pathways Regulating Protein Synthesis in the Rat Soleus Muscle in the Early Period of Gravitational Unloading. Neurosci Behav Physi 47, 359–365 (2017). https://doi.org/10.1007/s11055-017-0405-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-017-0405-3