Abstract
Purpose
Studies on the impact of tapered-cuff tracheal tubes on rates of microaspiration and ventilator-associated pneumonia (VAP) in intubated patients have reported conflicting results. The aim of this study was to determine the influence of this shape of tracheal cuff on abundant microaspiration of gastric contents in critically ill patients.
Methods
All patients intubated in the intensive care unit (ICU) and requiring mechanical ventilation for at least 48 h were eligible for this multicenter cluster-randomized controlled cross-over open-label study. The primary outcome was abundant microaspiration of gastric contents, defined by the presence of pepsin at significant level in >30% of tracheal aspirates. Quantitative measurement of pepsin and salivary amylase was performed in all tracheal aspirates during the 48 h following enrollment.
Results
A total of 326 patients were enrolled in the ten participating ICUs (162 in the PVC tapered-cuff group and 164 in the standard-cuff group). Patient characteristics were similar in the two study groups. The proportion of patients with abundant microaspiration of gastric contents was 53.5% in the tapered-cuff and 51.0% in the standard-cuff group (odds ratio 1.14, 95% CI 0.72–1.82). While abundant microaspiration of oropharyngeal secretions was not significantly different (77.4 vs 68.6%, p = 0.095), the proportion of patients with tracheobronchial colonization was significantly lower (29.6 vs 43.3%, p = 0.01) in the tapered-cuff than in the standard-cuff group. No significant difference between the two groups was found for other secondary outcomes, including ventilator-associated events and VAP.
Conclusions
This trial showed no significant impact of tapered-cuff tracheal tubes on abundant microaspiration of gastric contents.
Trial registration
ClinicalTrials.gov, number NCT01948635.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is a common ICU-acquired infection in patients requiring intubation and mechanical ventilation [1, 2]. This infection is associated with increased morbidity, mortality, and cost [3]. During the last decades, substantial progress in understanding VAP pathogenesis has been achieved [4, 5]. However, additional improvement in preventive strategies is still required to keep reducing VAP incidence and its negative impact on outcomes [6]. Potential means to achieve this goal include methods aiming at avoiding intubation or reducing its duration [7, 8]. Technological advances also brought new methods to decrease microaspiration of contaminated secretions in intubated patients [9–11].
The primary mechanism of VAP pathogenesis is microaspiration of gastric and oropharyngeal contaminated secretions [9, 12]. Whilst microaspiration of contaminated secretions around the tracheal cuff is a multifactorial process, tracheal cuff shape might play an important role in its occurrence [13]. Previous bench studies suggested a beneficial effect of tapered-cuff tracheal tubes in reducing leakage around the cuff, by providing a permanent sealing zone between the cuff and the tracheal wall [14, 15]. An animal study also reported significant reduction of leakage using polyvinyl chloride (PVC) tapered cuffs versus cylindrical cuffs [16]. However, other in vitro and animal studies did not confirm these findings [17, 18]. Recent clinical studies reported conflicting results on the impact of tapered-cuff tracheal tube on microaspiration, tracheobronchial colonization, early-onset postoperative pneumonia, and VAP [19–24]. Further, these studies presented several limitations, including observational design [19, 23], short-term evaluation [19–21], single-center design [19–21, 24], specific patient population [20, 21], use of inaccurate markers of microaspiration [20, 22], and incomplete evaluation of microaspiration [19, 21]. Therefore, no definite conclusion could be drawn regarding the impact of tapered-cuff tracheal tube on microaspiration and VAP.
We hypothesized that tapered-cuff tracheal tube would reduce abundant microaspiration of gastric contents and conducted a randomized controlled multicenter study to determine the impact of PVC tapered-cuff tracheal tube, compared with PVC standard-cuff tracheal tube, on abundant microaspiration of gastric contents in patients with a predicted duration of mechanical ventilation of at least 48 h.
Methods
Study design and participants
This multicenter cluster randomized cross-over controlled and open-label trial was conducted in 10 French mixed-ICUs during a 16-month period (from June 2014 to October 2015). The study protocol [25] was reviewed and approved by the Ethics Committee and Institutional Review Board of Lille University Hospital (approved by July 2, 2013, registration 2013 A00534 41). Written, informed consent to participate in the study was required from all patients, or their next of kin, before enrollment. When consent was given by proxies, the patient was informed as soon as possible, and his written consent was obtained. This trial is registered with ClinicalTrials.gov, number NCT01948635.
Patients older than 18 years who required intubation in the ICU with the study tracheal tube and have an expected duration of mechanical ventilation of at least 48 h after inclusion were eligible. Patients were excluded from the study if they were pregnant, contraindicated for enteral feeding, or intubated for longer than 72 h at screening for eligibility in the trial. Other exclusion criteria were tracheostomy at ICU admission, previous enrollment in this study, or inclusion in another study that may interfere with this trial.
Randomization and masking
Because tracheal intubation is an urgent procedure in ICU patients, the randomization was performed on the participating ICUs and not on the patients. The 10 ICUs were randomized into two balanced groups, according to a 1:1 assignment intervention sequence determined using a computerized random number generator and conducted by the Statistics Department at Lille University Hospital. Half of ICUs were randomized to use a PVC tapered cuff (Taper Guard®, Covidien, Athlone, Ireland) in the first 1-month period and a PVC standard cuff (Hi-Lo®, Covidien, Athlone, Ireland) in the second 1-month period. The other half of the ICUs used the two interventions in the reverse sequence. In all ICUs, the same order of cuff shape as that in the first 2 months was used for the subsequent months of the study. When reintubation was required, a tracheal tube with the same cuff shape as that in the first intubation was used.
Treatment allocation was open-label, as masking was not possible because of the obvious visible cuff shape difference during intubation. However, pepsin and salivary (i.e., alpha-) amylase, measurements were performed blindly, and VAP diagnosis was confirmed by two blinded physicians. Discordance between the two physicians was resolved by a third blinded investigator.
Procedures and definitions
Inclusion was performed at least 12 h after intubation. All tracheal aspirates were collected during the 48 h following enrollment in the trial to measure pepsin and salivary amylase. All tracheal aspirates were stored at −20 °C and sent to the central laboratory at Lille University Hospital, where all measurements were blindly performed (ELISA technique for pepsin and difference between total and pancreatic amylase activity for salivary amylase) [26, 27]. Abundant gastric microaspiration was defined by significant pepsin level (>200 ng/ml) in >30% of tracheal aspirates per patient [26]. Abundant oropharyngeal microaspiration was defined by significant salivary amylase level (>1685 IU/ml) in >30% of tracheal aspirates per patient [27, 28].
VAP was defined using clinical, radiographic, and microbiological criteria. Namely, a new and persistent infiltrate on the chest radiograph associated with two of the three following criteria: purulent tracheal aspirates, hyperthermia >38 °C, or hypothermia <36 °C, and peripheral leukocytosis >10 G/l or <1.5 G/l. In addition, microbiological confirmation was required using tracheal aspirate ≥105 CFU/ml or bronchoalveolar lavage ≥104 CFU/ml [29]. Tracheobronchial colonization was defined by positive (≥105 CFU/ml) tracheal aspirate without clinical or radiological signs of VAP. To diagnose tracheobronchial colonization, quantitative tracheal aspirate was performed after intubation, and two times a week.
Ventilator-associated events (VAE) were defined as sustained increase in ventilator support (minimum positive end-expiratory pressure (PEEP) increase ≥2.5 cmH2O or minimum FiO2 increase ≥15%) after ≥2 days of stable or decrease settings [30]. Patients with PEEP ≥ 7.5 cmH2O or FiO2 ≥ 70% during the first 3 days of mechanical ventilation were screened for VAE only if they subsequently stabilized and only required minimal ventilator support (PEEP ≤ 5 cmH2O, FiO2 ≤ 40% for ≥2 days).
All patients were prospectively followed to detect clinical, radiological, or microbiological signs of suspected VAP or VAE until day 28 or ICU discharge, whichever happens first.
In all participating ICUs, cuff pressure was checked with a manual manometer every 8 h and kept around 25 cmH2O. Patients were ventilated in a semirecumbent position. Female and male patients were intubated with tracheal tubes sized 7.5 and 8 mm, respectively. Head of bed elevation was checked every 3 h. Oropharyngeal decontamination was performed with 0.10% chlorhexidine every 3 h. Mechanical ventilation was performed with a PEEP of at least 5 cmH2O, unless contraindicated. Criteria for weaning from mechanical ventilation were checked every day to reduce the duration of tracheal intubation [31]. Systematic stress ulcer prophylaxis was not recommended in routine practice. The shortest duration of sedation was recommended, using a nurse-driven protocol. Tracheal suctioning was performed every 3 h, or more frequently if necessary, using an open system. Subglottic secretion drainage was not used, and respiratory circuit was not routinely changed in study patients.
Outcomes
The primary endpoint of this trial was the proportion of patients with abundant microaspiration of gastric contents, measured during the 48 h following inclusion.
The proportion of patients with abundant oropharyngeal microaspiration, measured during the 48 h following inclusion, was a secondary outcome. Other secondary outcomes included tracheobronchial colonization, VAP, VAE, ICU-acquired infection, antimicrobial-free days, invasive mechanical ventilation free-days, length of ICU stay, and proportion of patients who died in the ICU, from inclusion through day 28 or to ICU discharge, whichever happens first.
Statistical analysis
We calculated that a sample of 312 patients would provide a power of 80% to detect an absolute risk reduction in primary endpoint of 20% in the tapered-cuff group, with a two-sided type I error of 0.05, assuming a primary endpoint rate of 50% in the standard cuff group (control), a rate of 10% of patients without any tracheal secretions, and an intraclass correlation coefficient of 0.035 [25].
All analyses were performed in all randomized patients on the basis of their original group of randomization, according to the intention-to-treat principle. Qualitative variables were expressed as frequencies and percentages. Quantitative variables were expressed as mean (standard deviation) or median (interquartile range). Normality of distribution was assessed graphically and using the Shapiro–Wilk test. Quantitative variables were compared between the two groups using Student’s t test, or Mann–Whitney U test for variables with non-Gaussian distribution. Qualitative variables were compared using Chi-square test, or Fisher’s exact test when the expected cell frequency was less than 5.
The difference in rate of abundant microaspiration of gastric contents study groups was calculated as absolute and relative risk reduction (tapered versus standard cuff) with 95% confidence interval.
In order to take into account the cluster effect, comparison of primary endpoint was performed using a mixed logistic regression model including center as random effect. Center-adjusted odds ratio (OR) was derived from this model. Missing data for the primary endpoint were treated by multiple imputation, using regression-switching approach.
Secondary binary endpoints were compared between the two groups using a mixed logistic regression model including center as random effect. Secondary quantitative endpoints were all non-normally distributed and were compared using Mann–Whitney U test.
Further details on methods are presented in the online supplementary material.
Results
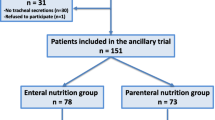
A total of 326 medical patients were randomized in the 10 participating ICUs and were all included in the final analysis (Fig. 1). Pepsin and salivary amylase were quantitatively measured in 2739 tracheal aspirates coming from 303 patients. No tracheal secretions were present in 23 (7.3%) patients. The median number of tracheal aspirates per patient in which pepsin and salivary amylase were measured was similar in the two groups.
Patient characteristics
Patient characteristics at ICU admission and at inclusion were similar in the two groups (Table 1). No significant difference was found between the two groups in patient characteristics during the 48 h following inclusion or during ICU stay (Table 2).
Outcomes
No significant difference was found between the two groups regarding the primary outcome (Table 3). No significant difference was found in abundant microaspiration of oropharyngeal secretions between the two groups. The percentage of patients with tracheobronchial colonization was significantly lower in the tapered cuff group compared with the standard cuff group. However, no significant difference was found in bacterial concentration in colonized patients between the two groups (Table 4). No significant difference was found in other secondary outcomes, including percentage of patients with VAP, VAE, other ICU-acquired infections, mechanical ventilation-free days, antibiotic-free days, ICU length of stay, or ICU mortality (Table 4).
Percentage of tracheal aspirates positive for pepsin or salivary amylase was similar in the two study groups; this was also true for mean pepsin and amylase levels (Table 5).
Discussion
In this trial we found that PVC tapered-cuff tracheal tube, compared with PVC standard-cuff tracheal tube, did not reduce abundant microaspiration of gastric contents in intubated critically ill patients. No significant impact of tapered-cuff tracheal tube was found on abundant microaspiration of oropharyngeal secretions, bacterial tracheobronchial concentration, VAP, VAE, ICU-acquired infection, antibiotic-free days, mechanical ventilation-free days, ICU length of stay, or ICU mortality. Tapered-cuff tracheal tube was associated with reduced percentage of patients with tracheobronchial colonization.
Strengths of our study are the multicenter randomized controlled design and the careful evaluation of microaspiration of gastric contents and oropharyngeal secretions, using quantitative pepsin and salivary amylase measurement in all tracheal aspirates for 48 h. Two previous randomized controlled trials [21, 22] evaluated the impact of tapered-shaped tracheal cuff on microaspiration, tracheobronchial colonization, early postoperative pneumonia, and VAP in critically ill patients. In the large TOPCuff randomized controlled multicenter trial [22], the impact of polyurethane (versus PVC) and of tapered cuff (compared with cylindrical cuff) on tracheobronchial colonization was evaluated. No significant difference was found in tracheobronchial colonization or VAP incidence between the different study groups. Several differences with our study should be outlined. Whilst the comparator of tapered cuff was cylindrical cuff in the TOPCuff study, it was standard cuff in ours. The primary outcome of that study, i.e., tracheobronchial colonization at day 3, was different from the one used in ours. This outcome might have been influenced by the common use of antimicrobials in study patients, and potential exogenous contamination of airway circuit and tracheal tubes. In the single-center randomized controlled TETRIS study [21], Monsel and colleagues aimed to evaluate the impact of tapered-cuff compared with standard-cuff tracheal tube on postoperative pneumonia and microaspiration. No significant impact of this intervention was found on primary or secondary outcomes. As acknowledged by the authors, the single-center design and inclusion of only patients after major vascular surgery preclude definite conclusions. In addition, pepsin and salivary amylase were only measured at two time points.
One potential explanation for the absence of beneficial effect of tapered-cuff tracheal tube on the percentage of patients with abundant microaspiration of gastric contents is the reduced contact zone between the cuff and the tracheal wall, which might result in increased mobility of tapered-cuff compared with standard-cuff tracheal tube. Movement of the tracheal tube has been previously reported to be a risk factor for leakage and microaspiration in in vitro and clinical studies [32, 33]. This might explain why in vitro [14, 15], animal [16], and one randomized controlled clinical study [20] found decreased leakage around the cuff using tapered-cuff compared with standard-cuff tracheal tube. Indeed, the randomized controlled clinical trial was performed in patients heavily anesthetized for lumbar surgery, and the mobility of the tracheal tube was probably very limited [20]. Similarly, animals were anesthetized and paralyzed in the study performed by Lichtenthal and colleagues [16], and the tracheal tubes were systematically fixed in bench studies [14, 15].
Whilst no significant impact of tapered cuff was found on abundant microaspiration and VAP, the percentage of patients with tracheobronchial colonization was significantly lower in the tapered-cuff group compared with standard-cuff group. Previous studies suggested that tracheobronchial colonization was a risk factor for subsequent VAP [34]. In addition, a continuum between microaspiration, colonization, and lower respiratory tract infections was reported [4, 35]. Therefore, the significant reduction in the rate of patients with tracheobronchial colonization could be beneficial in these patients. However, no significant difference was found in bacterial concentration or in VAP rates between the two groups. The discrepancy between the significant difference in percentage of patients with tracheobronchial colonization and the absence of significant difference in VAP or VAE rates could be explained by the lower incidence of VAP and VAE compared with colonization. However, it should be outlined that tracheobronchial colonization was a secondary outcome. Further, microaspiration of endogenous bacteria coming from the subglottic area could not be differentiated from exogenous bacteria coming from ventilator circuit and tracheal tube manipulations, which might have influenced the relationship between microaspiration and colonization.
Other potential explanations for the discrepancy between the absence of significant difference in abundant microaspiration of gastric and oropharyngeal secretions and the significant difference in tracheobronchial colonization rate between the two groups include the relatively short period of evaluation of microaspiration and the definition used for microaspiration. Quantitative measurement of pepsin and salivary amylase was performed during a 48 h-period. Whilst a large number of tracheal aspirates were analyzed, the 48 h-period represented approximately 20% of the whole duration of mechanical ventilation in study patients. The definition used of abundant microaspiration of gastric contents (>30% of tracheal aspirates positive for pepsin) was different from the one used in our previous studies [26, 27]. However, this definition was based on the results of a large amount of tracheal aspirates, coming from a large number of patients included in a previous randomized controlled trial [26] and in an unpublished study. To the best of our knowledge, no other studies have evaluated other thresholds to define abundant microaspiration using quantitative pepsin and salivary amylase levels in consecutive samples from intubated critically ill patients.
Subglottic secretion drainage and continuous control of cuff pressure were not used in study patients. Several recent studies showed that subglottic secretion drainage was efficient in preventing VAP [36]. However, when our study was designed and started, the evidence in favor of this preventive measure was less strong. Continuous control of cuff pressure could be interesting to prevent VAP [26, 37]. However, this measure is not currently recommended, because of the low level of evidence for its use [38].
In addition to the limitations discussed above, we designed an open-label study, because blinding ICU physicians was not feasible during intubation. However, we estimate this bias to be minor, as the primary endpoint was objective and blindly assessed by physicians who performed pepsin and amylase measurement. Second, randomization was performed by center and not by patient to avoid delaying intubation or excluding patients with urgent intubation. Nevertheless, the number of patients included in the two study groups was finally similar, as were their characteristics. Third, we did not evaluate tracheal ischemic lesions or stridor in study patients. Although no clinical data are available on the relationship between tapered cuff and tracheal ischemic lesions, tapered cuff might result in more severe lesions, as the pressure is applied to a smaller tracheal surface. Fourth, the incidence of VAE was lower than VAP incidence, and VAE incidence reported in previous studies [39]. One potential explanation is the high percentage of patients with high ventilator setting dependence. Further, we used the Centers for Disease Control (CDC) definition for VAE, but not for VAP. The use of quantitative cultures to define VAP in our study probably resulted in lower incidence of VAP, compared with the CDC definition. Fifth, only a small proportion of screened patients were included in this study. However, two-thirds of screened patients were excluded because intubation was performed before ICU admission, which is in line with real life in French ICUs. Finally, the adherence to all VAP prevention recommendations was not measured in different ICUs. However, similar VAP preventive measures were recommended in the participating ICUs.
Conclusion
In this multicenter randomized trial, tapered-cuff tracheal tube was not superior to standard-cuff tracheal tube in reducing microaspiration of gastric contents. Our results suggest that tapered-cuff tracheal tube should not be used to prevent microaspiration or VAP in ICU patients.
References
Nair GB, Niederman MS (2014) Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 41:34–48. doi:10.1007/s00134-014-3564-5
Vincent J-L, Bassetti M, François B et al (2016) Advances in antibiotic therapy in the critically ill. Crit Care 20:133. doi:10.1186/s13054-016-1285-6
Melsen WG, Rovers MM, Groenwold RHH et al (2013) Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 13:665–671. doi:10.1016/S1473-3099(13)70081-1
Martin-Loeches I, Povoa P, Rodríguez A et al (2015) Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med 3:859–868. doi:10.1016/S2213-2600(15)00326-4
Blot SI, Rello J, Koulenti D (2016) The value of polyurethane-cuffed endotracheal tubes to reduce microaspiration and intubation-related pneumonia: a systematic review of laboratory and clinical studies. Crit Care 20:203. doi:10.1186/s13054-016-1380-8
Guillamet CV, Kollef MH (2015) Ventilator associated pneumonia in the ICU: where has it gone? Curr Opin Pulm Med 21:226–231. doi:10.1097/MCP.0000000000000151
Papazian L, Corley A, Hess D et al (2016) Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. doi:10.1007/s00134-016-4277-8
Kalil AC, Metersky ML, Klompas M et al (2016) Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:575–582. doi:10.1093/cid/ciw504
Nseir S, Zerimech F, Jaillette E et al (2011) Microaspiration in intubated critically ill patients: diagnosis and prevention. Infect Disord Drug Targets 11:413–423
Blot SI, Poelaert J, Kollef M (2014) How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infect Dis 14:119. doi:10.1186/1471-2334-14-119
Lacherade J-C, De Jonghe B, Guezennec P et al (2010) Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med 182:910–917. doi:10.1164/rccm.200906-0838OC
Jaillette E, Martin-Loeches I, Artigas A, Nseir S (2014) Optimal care and design of the tracheal cuff in the critically ill patient. Ann Intensive Care 4:7. doi:10.1186/2110-5820-4-7
Branson RD, Hess DR (2015) Lost in translation: failure of tracheal tube modifications to impact ventilator-associated pneumonia. Am J Respir Crit Care Med 191:606–608. doi:10.1164/rccm.201502-0206ED
Dave MH, Frotzler A, Spielmann N et al (2010) Effect of tracheal tube cuff shape on fluid leakage across the cuff: an in vitro study. Br J Anaesth 105:538–543. doi:10.1093/bja/aeq202
Madjdpour C, Mauch J, Dave MH et al (2012) Comparison of air-sealing characteristics of tapered- vs. cylindrical-shaped high-volume, low-pressure tube cuffs. Acta Anaesthesiol Scand 56:230–235. doi:10.1111/j.1399-6576.2011.02542.x
Lichtenthal P, Borg U, Maul D (2010) Do endotracheal tubes prevent microaspiration? Crit Care 14:P229. doi:10.1186/cc8461
Li Bassi G, Ranzani OT, Marti JD et al (2013) An in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressures. Crit Care Med 41:518–526. doi:10.1097/CCM.0b013e31826a4804
Li Bassi G, Luque N, Martí JD et al (2015) Endotracheal tubes for critically ill patients. Chest 147:1327–1335. doi:10.1378/chest.14-1438
Nseir S, Zerimech F, De Jonckheere J et al (2010) Impact of polyurethane on variations in tracheal cuff pressure in critically ill patients: a prospective observational study. Intensive Care Med 36:1156–1163. doi:10.1007/s00134-010-1892-7
D’Haese J, De Keukeleire T, Remory I et al (2013) Assessment of intraoperative microaspiration: does a modified cuff shape improve sealing? Acta Anaesthesiol Scand 57:873–880. doi:10.1111/aas.12119
Monsel A, Lu Q, Le Corre M et al (2016) Tapered-cuff endotracheal tube does not prevent early postoperative pneumonia compared with spherical-cuff endotracheal tube after major vascular surgery: a randomized controlled trial. Anesthesiology. doi:10.1097/ALN.0000000000001053
Philippart F, Gaudry S, Quinquis L et al (2015) Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am J Respir Crit Care Med 191:637–645. doi:10.1164/rccm.201408-1398OC
Bowton DL, Hite RD, Martin RS, Sherertz R (2013) The impact of hospital-wide use of a tapered-cuff endotracheal tube on the incidence of ventilator-associated pneumonia. Respir Care 58:1582–1587. doi:10.4187/respcare.02278
Jaillette E, Zerimech F, De Jonckheere J et al (2013) Efficiency of a pneumatic device in controlling cuff pressure of polyurethane-cuffed tracheal tubes: a randomized controlled study. BMC Anesthesiol 13:50. doi:10.1186/1471-2253-13-50
Jaillette E, Brunin G, Girault C et al (2015) Impact of tracheal cuff shape on microaspiration of gastric contents in intubated critically ill patients: study protocol for a randomized controlled trial. Trials 16:429. doi:10.1186/s13063-015-0955-z
Nseir S, Zerimech F, Fournier C et al (2011) Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med 184:1041–1047. doi:10.1164/rccm.201104-0630OC
Dewavrin F, Zerimech F, Boyer A et al (2014) Accuracy of alpha amylase in diagnosing microaspiration in intubated critically-ill patients. PLoS One 9:e90851. doi:10.1371/journal.pone.0090851
Filloux B, Bedel A, Nseir S et al (2013) Tracheal amylase dosage as a marker for microaspiration: a pilot study. Miner Anestesiol 79:1003–1010
American Thoracic Society, Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST
Klompas M, Khan Y, Kleinman K et al (2011) Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One 6:e18062. doi:10.1371/journal.pone.0018062
Boles J-M, Bion J, Connors A et al (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056. doi:10.1183/09031936.00010206
Lizy C, Swinnen W, Labeau S et al (2014) Cuff pressure of endotracheal tubes after changes in body position in critically ill patients treated with mechanical ventilation. Am J Crit Care 23:e1–e8. doi:10.4037/ajcc2014489
Chenelle CT, Oto J, Sulemanji D et al (2015) Evaluation of an automated endotracheal tube cuff controller during simulated mechanical ventilation. Respir Care 60:183–190. doi:10.4187/respcare.03387
Paling FP, Wolkewitz M, Bode LGM et al (2016) Staphylococcus aureus colonization at ICU admission as a risk factor for developing S. aureus ICU pneumonia. Clin Microbiol Infect 23(1):49.e9–49.e14. doi:10.1016/j.cmi.2016.09.022
Nseir S, Povoa P, Salluh J et al (2016) Is there a continuum between ventilator-associated tracheobronchitis and ventilator-associated pneumonia? Intensive Care Med. doi:10.1007/s00134-016-4283-x
Mao Z, Gao L, Wang G et al (2016) Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit Care 20:353. doi:10.1186/s13054-016-1527-7
Nseir S, Lorente L, Ferrer M et al (2015) Continuous control of tracheal cuff pressure for VAP prevention: a collaborative meta-analysis of individual participant data. Ann Intensive Care 5:43. doi:10.1186/s13613-015-0087-3
Klompas M, Branson R, Eichenwald EC et al (2014) Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35:915–936. doi:10.1086/677144
Magill SS, Li Q, Gross C et al (2016) Incidence and characteristics of ventilator-associated events reported to the National Healthcare Safety Network in 2014. Crit Care Med 44:2154–2162. doi:10.1097/CCM.0000000000001871
Acknowledgements
In addition to the authors of the manuscript, the BestCuff Study Group includes the following investigators and collaborators:
Geoffrey Ledoux, Sébastien Preau, Mercé Jourdain, Ahmed Elkalioubie, Julien Poissy, Patrick Girardie, Amélie Cerf (Centre de Réanimation, CHU Lille, rue E. Laine, 59037 Lille Cedex, France).
Réginald Pordes, Pierre Ducq, Halim Mahfoudi (Réanimation Polyvalente, CH Dr Duchenne, allée Jacques Monod, BP 609, 62321 Boulogne-Sur-Mer, France).
Antoine Marchalot, Gioia Gastaldi, Pauline Bernier-Enguerrand (Réanimation Médicale, Hôpital C. Nicolle, 1 rue de Germont, 76031 Rouen Cedex, France).
Malika Balduyck, Patrice Maboudou (Université de Lille et Pôle de Biologie Pathologie Génétique du CHRU de Lille, Laboratoire de Biochimie et Biologie Moléculaire, 59000, Lille, France).
Pierre-Yves Delannoy, Christophe Muller, Olivier Leroy (Réanimation Médicale et Infectieuse, CH de Tourcoing, 115 rue du Président Coty, 59208 Tourcoing Cedex, France).
Delphine Colling, Patrick Herbecq, (Réanimation Polyvalente, Hôpital Victor Provo, 17 bd Lacordaire, BP 359, 59056 Roubaix, France).
Amélie Mazaud, Sébastien Béague (Service de réanimation polyvalente, 130 Avenue Louis Herbeaux BP 6367, 59140 Dunkerque, France).
Thierry Van Der Linden, Philippe Cabaret (Réanimation Polyvalente, CH Saint Philibert, 115 Rue du Grand But, BP 249, 59462 Lomme Cedex, France).
Florent Dewavrin, Virginie Mignaux (Réanimation Médicale, CH de Valenciennes, Avenue Desandrouin, BP 479, 59322 Valenciennes Cedex, France).
Johanna Temime, Didier Thevenin (Réanimation Polyvalente, CH Dr Schaffner, 99 route de La Bassée, BP 8, 62307 Lens Cedex, France).
Author’s contribution
EJ and SN designed the study and wrote the manuscript. HB, JL, and AD wrote the statistical analysis plan and performed all statistical analyses. FZ performed pepsin and amylase measurements. EJ, GB, CG, AC, CBD, CF, FM, IA, SB, LR, FT, ED DT, and CD contributed to acquisition of the study data. All authors revised the manuscript for important intellectual content, and all authors read and approved the final manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
SN has received lecture fees from Medtronic and MSD, and he is a member of the advisory boards of Ciel Medical and Bayer. Other authors have no conflicts of interest to declare.
Funding
This trial was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique Interrégional; N° 2012_45). Covidien donated the tracheal tubes used in this study. Neither the French Ministry of Health nor Covidien had any role in the design or conduct of the study. They did not have any role in the preparation, review, or approval of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for decision to submit for publication.
Additional information
The members of the BestCuff Study Group and the BoRéal Network are listed in the “Acknowledgements” and ESM2.
Take-home message: Tapered-cuff tracheal tubes are not superior to standard-cuff tracheal tubes in reducing microaspiration of gastric contents. Our results suggest that tapered-cuff tracheal tubes should not be used to prevent microaspiration or VAP in ICU patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jaillette, E., Girault, C., Brunin, G. et al. Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: a multicenter cluster-randomized cross-over controlled trial. Intensive Care Med 43, 1562–1571 (2017). https://doi.org/10.1007/s00134-017-4736-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4736-x