Abstract
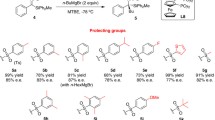
Chiral N-(tert-butyl)sulfinyl aldimines easily prepared from commercially available compounds have been used as starting materials for the following processes: (i) hydrogen transfer, (ii) addition of zincates, (iii) In-promoted allylation, and (iv) addition of zinc enolates. In all cases, the final desulfinylation to yield the expected chiral α-substituted primary amines was easily performed with hydrochloric acid in an organic solvent. This methodology has been successfully applied to the preparation of chiral natural products such as alkaloids and amino acids.
Similar content being viewed by others
References
MDL Drug Data Report, MDL Information Systems, Inc., CA.
D. A. Cogan, G. Liu, J. Ellman, Tetrahedron, 1999, 55, 8883
J. A. Ellman, T. D. Owens, T. P. Tang, Acc. Chem. Res., 2002, 35, 984
J. A. Ellman, Pure Appl. Chem., 2003, 75, 39.
R. Noyori, S. Hashiguchi, Acc. Chem. Res., 1997, 30, 97
M. J. Palmer, M. Wills, Tetrahedron: Asymmetry, 1999, 10, 2045
C. Wang, X. Wu, J. Xiao, Chem. Asian J., 2008, 3, 1750
F. Foubelo, C. Nájera, M. Yus, Tetrahedron: Asymmetry, 2015, 26, 769.
S. Kobayashi, H. Ishitani, Chem. Rev., 1999, 99, 1069
G. Helmchen, A. Pfaltz, Acc. Chem. Res., 2000, 33, 336
M. Wills, in Modern Reduction Methods, Eds P. G. Andersson, I. J. Munslow, Wiley-VCH, Weinheim, 2008, p. 271
F. Foubelo, M. Yus, Chem. Rec., 2015, 15, 907.
G. Liu, D. A. Cogan, T. D. Owens, T. P. Tang, J. A. Ellman, J. Org. Chem., 1999, 64, 1278
J. F. Collados, E. Toledano, D. Guijarro, M. Yus, J. Org. Chem., 2012, 77, 5744.
M. Wills, M. Palmer, A. Smith, J. Kenny, T. Walsgrove, Molecules, 2000, 5, 4.
D. Guijarro, O. Pablo, M. Yus, Tetrahedron Lett., 2009, 50, 5386
D. Guijarro, O. Pablo, M. Yus, J. Org. Chem., 2010, 75, 5265 [Synfacts, 2010, 1254]
D. Guijarro, O. Pablo, M. Yus, Tetrahedron Lett., 2011, 52, 789 [Synfacts, 2011, 508]
O. Pablo, D. Guijarro, G. Kovács, A. Lleds, G. Ujaque, M. Yus, Chem. Eur. J., 2012, 18, 1969
D. Guijarro, O. Pablo, M. Yus, Org. Synth., 2013, 90, 338.
D. Guijarro, O. Pablo, M. Yus, J. Org. Chem., 2013, 78, 3647.
O. Pablo, D. Guijarro, M. Yus, J. Org. Chem., 2013, 78, 9181.aaa
M. Yus, D. J. Ramón, Recent Res. Dev. Org. Chem., 2002, 6, 297.
D. J. Ramón, M. Yus, Angew. Chem., Int. Ed., 2004, 43, 284.
R. Almansa, D. Guijarro, M. Yus, Tetrahedron: Asymmetry, 2008, 19, 603
R. Almansa, D. Guijarro, M. Yus, Tetrahedron: Asymmetry, 2008, 19, 2484.
R. Almansa, D. Guijarro, M. Yus, Tetrahedron Lett., 2009, 50, 3198.
R. Almansa, D. Guijarro, M. Yus, Tetrahedron Lett., 2009, 50, 4190
R. Almansa, J. F. Collados, D. Guijarro, M. Yus, Tetrahedron: Asymmetry., 2010, 21, 1421.
S. Kobayashi, H. Ishitani, Chem. Rev., 1999, 99, 1069
G. Alvaro, D. Savoia, Synlett, 2002, 651.
M. Yus, J. C. González-Gómez, F. Foubelo, Chem. Rev., 2011, 111, 7774
M. Yus, J. C. González-Gómez, F. Foubelo, Chem. Rev., 2013, 113, 5595.
F. Foubelo, M. Yus, Tetrahedron: Asymmetry, 2004, 15, 3823
J. C. González-Gómez, M. Medjahdi, F. Foubelo, M. Yus, J. Org. Chem., 2010, 75, 6308.
F. Foubelo, M. Yus, Eur. J. Org. Chem., 2014, 485.
D. J. Ramón, M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602.
J. C. González-Gómez, F. Foubelo, M. Yus, Org. Synth., 2012, 89, 88.
J. C. González-Gómez, F. Foubelo, M. Yus, Synlett, 2008, 2777.
M. Medjahdi, J. C. González-Gómez, F. Foubelo, M. Yus, Heterocycles, 2012, 86, 727.
S. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Forrow, E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 1307.
M. Medjahdi, J. C. González-Gómez, F. Foubelo, M. Yus, Heterocycles, 2008, 76, 569
M. Medjahdi, J. C. González-Gómez, F. Foubelo, M. Yus, J. Org. Chem., 2009, 74, 7859.
H. K. Dema, F. Foubelo, M. Yus, Heterocycles, 2010, 80, 125
H. K. Dema, F. Foubelo, M. Yus, Heterocycles, 2011, 82, 1411.
I. Bosque, J. C. González-Gómez, F. Foubelo, M. Yus, J. Org. Chem., 2012, 77, 780
J. C. Bosque, J. C. González-Gómez, F. Foubelo, M. Yus, J. Org. Chem., 2012, 77, 4190.
I. Bosque, J. C. González-Gómez, A. Guijarro, F. Foubelo, M. Yus, J. Org. Chem., 2012, 77, 10340.
M. Medjahdi, J. C. González-Gómez, F. Foubelo, M. Yus, Eur. J. Org. Chem., 2011, 2230.
J. A. Sirvent, F. Foubelo, M. Yus, Heterocycles, 2014, 88, 1163.
J. A. Sirvent, F. Foubelo, M. Yus, J. Org. Chem., 2014, 79, 1356.
J. A. Sirvent, F. Foubelo, M. Yus, Chem. Commun., 2012, 48, 2543.
J. A. Sirvent, F. Foubelo, M. Yus, Eur. J. Org. Chem., 2013, 2461.
M. Kitamura, T. Miki, K. Nakano, R. Noyori, Tetrahedron Lett., 1996, 37, 5141
B. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos, A. H. de Vries, Angew. Chem., Int. Ed., 1997, 36, 2620
A. Alexakis, C. Benhaim, Eur. J. Org. Chem., 2002, 3221.
J. C. González-Gómez, F. Foubelo, M. Yus, Tetrahedron Lett., 2008, 49, 2343 [Synfacts, 2009, 2547]
J. C. González-Gómez, F. Foubelo, M. Yus, J. Org. Chem., 2009, 74, 2547.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences, Professor I. P. Beletskaya.
This review is based on the presentation by one of the authors (M. Yus) at the International Congress on Heterocyclic Chemistry “KOST-2015” (October 18–23, 2015, Moscow, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1667–1686, July, 2016.
Rights and permissions
About this article
Cite this article
Foubelo, F., Yus, M. Recent advances in the use of chiral aldimines in asymmetric synthesis. Russ Chem Bull 65, 1667–1686 (2016). https://doi.org/10.1007/s11172-016-1496-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1496-7