Abstract
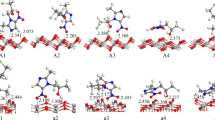
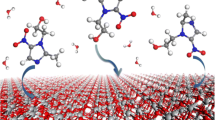
Density functional theory (DFT) method, considering periodic boundary conditions (PBC) and full geometry optimization, was used to study the adsorption of different tautomers of 1,3-thiazol-2-amine on TiO2 (101) and (001) anatase surfaces. The optimized structures of the tautomers on the surface and their corresponding adsorption energies (E ad) were determined. The tautomers were adsorbed on the surfaces mostly through the interaction between Ti atom on the surface and the lone electron pairs of their N, N3, and S atoms. The adsorption of tautomers through their N sites on the surfaces was more favorable compared to N3 and S sites. The adsorption of tautomers on (001) surface was more favorable than their adsorption on (101) surface. The adsorption of 1,3-thiazol-2-amine decreased the band gap of TiO2 which is favorable for solar cells. Comparison of the calculated total density of states (TDOS) of TiO2 + adsorbate with that of bare TiO2 showed the presence of the extra peaks in first band gap of TiO2 in the valence region which increased the conductivity of the surface. Also, for the adsorption of some tautomers, extra states were seen in the second band gap of TiO2 between the valence and conduction band of TiO2. The adsorption of the tautomers shifted the TDOS of the surface to the lower energy compared to the bare surface and caused a negative shift in the TiO2 Fermi level. The effect of solvent on the adsorption of the tautomers on the surfaces was also studied.







Similar content being viewed by others
References
Park H, Oub H-H, Kang U, Choi J, Hoffmann MR (2016) Catal Today 266:153–159
Bellardita M, García-López EI, Marcì Megna GB, Pomillaa FR, Palmisanoa L (2015) RSC Adv 5:59037
Goh GKL, Chan KYS, Huang GS, Tay QL (2011) Australian J Chem 64:1235
Li Q, Jiang Z, Qin J, Li Z (2012) Australian J Chem 65:1203
Huang F, Cheng YB, Caruso RA (2012) Australian J Chem 64:820
Tricot F, Vocanson F, Chaussy D, Beneventi D, Party M, Destouches N (2015) RSC Adv 5:84560
Karunagaran B, Uthirakumar P, Chung SJ, Velumani S, Suh EK (2007) Mater Charact 58:680
Zhang Z, Zhou Y, Zhang S, Xu C (2006) Energy Fuel 20:2293
Schacht P, Hernández G, Cedeño L, Mendoza JH, Ramírez S, García L, Ancheyta J (2003) Energy Fuel 17:81
Liu Z, Zhang L, Jiang J, Bian C, Zhang Z, Gao Z (2013) Adv Chem Eng Sci 3:36
Rodriguez JA, Liu P, Stacchiola DJ, Senanayake SD, White MG, Chen JG (2015) ACS Cata 5:6696
Nolan M (2011) Chem Commun 47:8617
Galhenage RP, Yan H, Tenney SA, Park N, Henkelman G, Albrecht P, Mullins DR, Chen DA (2013) J Phys Chem C 117:7191
Augustynski J (1993) Electrochim Acta 38:43
Zhang H, Banfield JF (2002) Chem Mater 14:4145
Barnard AS, Zapol P (2004) Phys Rev B 70:235403
Barnard SA, Zapol P, Curtiss LA (2005) Sur Sci 582:173
Arrouvel C, Digne M, Breysse M, Toulhoat H, Raybaud P (2004) J Cata 222:152
Lazzeri M, Vittadini A, Selloni A (2001) Phys Rev B 63:155409
Vittadini A, Selloni A, Rotzinger FP, Grätzel M (1998) Phys Rev Lett 81:2954
Ohno T, Sarukawa K, Tokieda K, Matsumura M (2001) J Cata 203:82
Bredow T, Jug K (1995) J Phy Chem 99:285
Gong XQ, Selloni A (2005) J Phys Chem B 109:19560
Li W, Liang R, Hu A, Huang Z, Zhou YN (2014) RSC Adv 4:36959
Gratzel M (1991) Comm Inorg Chem 12:93
Boschloo G, Fitzmaurice D (1999) J Phys Chem B 103:2228
Gratzel M (2004) J Photochem Photobiol A Chem 164:3
Barbe CJ, Arendse F, Comte P, Jirousek M, Lenzmann F, Shklover V, Gratzel M (1997) J Am Ceram Soc 80:3157
Nakada S, Matsuda M, Kambe S, Saito Y, Kitamura T, Sakata T, Wada Y, Mori H, Yanagida S (2002) J Phys Chem B 106:10004
Kim H, Auyeung RCY, Ollinger M, Kushto GP, Kafafi ZH, Pique A (2006) Appl Phys A Mater Sci Process 83:73
Lavrencic Stangar U, Orel B, Neumann B (2003) Sol–Gel Sci Technol 26:1113
Brooks MR, Guo Z (2006) J Phys Chem B 110:15932
Guo J, Watanabe S, Janik MJ, Ma X, Song C (2010) Catal Today 149:218
Tian FH, Wang X, Zhao W, Zhao L, Chu T, Yu S (2013) Sur Sci 616:76
Nilsing M, Persson P, Ojamae L (2005) Chem Phys Lett 415:375
Bermudez VM (2010) Surf Sci 604:706
Zhang X, Chen Q, Hu W, Zhang J (2013) Appl Sur Sci 286:47
Shukri G, Kasai H (2014) Surf Sci 619:59
Xu Y, Chen WK, Liu SH, Cao MJ, Li JQ (2007) Chem Phys 331:275
Najafi Chermahini A, Hosseinzadeh B, Beni AS, Teimouri A, Moradi M (2014) J Mol Model 20:2086
Najafi Chermahini A, Farrokhpour H, Zeinodini A (2016) J Mol Struc 1121:203
Eicher T, Hauptmann S (2003) The chemistry of heterocycles. ISBN 3-527, 30720
Delley B (1990) J Chem Phys 92:508
Kim E, Weck PF, Berber S, Tomanek D (2008) Phys Rev B 78:113404
Benedek NA, Snook IK, Latham K, Yarovsky I (2005) Chem Phys 122:144102
Inada Y, Orita H (2008) J Comput Chem 29:225
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671
Klamt A, Schüürmann G (1993) J Chem Soc (Perkin Trans2) 2:799
Acknowledgements
The authors thank the Isfahan University of Technology (IUT) for its financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 8440 kb).
Rights and permissions
About this article
Cite this article
Farrokhpour, H., Vazifeh, M. & Najafi Chermahini, A. Adsorption modes of 1,3-thiazol-2-amine on the TiO2 (001) and (101) anatase surfaces. Struct Chem 28, 1151–1162 (2017). https://doi.org/10.1007/s11224-017-0920-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0920-4