Abstract
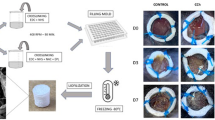
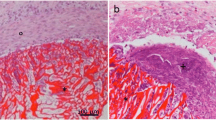
Natural materials such as collagen and alginate have promising applications as dural graft substitutes. These materials are able to restore the dural defect and create optimal conditions for the development of connective tissue at the site of injury. A promising material for biomedical applications is chitosan—a linear polysaccharide obtained by the deacetylation of chitin. It has been found to be nontoxic, biodegradable, biofunctional and biocompatible in addition to having antimicrobial characteristics. In this study we designed new chitin–chitosan substitutes for dura mater closure and evaluated their effectiveness and safety. Chitosan films were produced from 3 % of chitosan (molar mass—200, 500 or 700 kDa, deacetylation rate 80–90%) with addition of 20% of chitin. Antimicrobial effictively and cell viability were analysed for the different molar masses of chitosan. The film containing chitosan of molar mass 200 kDa, had the best antimicrobial and biological activity and was successfully used for experimental duraplasty in an in vivo model. In conclusion the chitin–chitosan membrane designed here met the requirements for a dura matter graft exhibiting the ability to support cell growth, inhibit microbial growth and biodegradade at an appropriate rate. Therefore this is a promising material for clinical duroplasty.






Similar content being viewed by others
References
Fontana R, Talamonti G, D’Angelo V, et al. Spontaneous haematoma as unusual complication of silasticdural substitute. Report of 2 cases. Acta Neurochir (Wien). 1992;115:64–6.
Van Calenberg F, Quintens E, Sciot R, et al. The use of Vicryl as a dura substitute: a clinical review of 78 surgical cases. Acta Neurochir (Wien). 1997;139:120–3.
Esposito F, Fusco PCM, Cavallo LM, et al. Collagen-only biomatrix as a novel dural substitute. Examination of the efficacy, safety and outcome: clinical experience on a series of 208 patients. Clin Neurol Neurosurg. 2008;110:343–51.
Knopp U, Christmann F, Reusche E, Sepehrnia A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects: a comparative animal experimental study. Acta Neurochir (Wien). 2005;147:877–87.
Beach HHA. Compound comminute fractures of the skull: epilepsy for five years, operation, recovery. Boston Med Surg J. 1890;122:313–5.
Barbolt TA, Odin M, Leger M, et al. Biocompatibility evaluation of dura mater substitutes in an animal model. Neurol Res. 2001;23:813–20.
Barth M, Tuettenberg J, Thome C, et al. Watertight dural closure: is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery. 2008;63:352–8.
Laun A, Tonn JC, Jerusalem C. Comparative study of lyophilized human dura mater and lyophilized bovine pericardium as dural substitutes in neurosurgery. Acta Neurochir (Wien). 1990;107:16–21.
Wang HT, Erdmann D, Olbrich KC, et al. Freeflap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg. 2007;119:865–72.
Yamada K, Miyamoto S, Takayama M, et al. Clinical application of a new bioasorbable artificial dura mater. J Neurosurg. 2002;96:731–5.
Kataoka K, Suzuki Y, Kitada M, et al. Alginate, a bioresorbable material derived from brown seaweed, enhances elongation of amputated axons of spinal cord in infant rats. J Biomed Mater Res. 2001;54:373–84.
Suzuki K, Suzuki Y, Ohnishi K, et al. Regeneration of transected spinal cord in young adult rats using freeze-dried alginate gel. Neuroreport. 1999;10:2891–94.
Jendelova P, Lesny P, Hejcl A, et al. The implantation of biodegradable macroporous polymer hydrogels into the injured rat spinal cord. Exp Neurol. 2005;193(1):1189
Lavik E, Teng YD, Snyder E, Langer R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods Mol Biol. 2002;198:89–97.
Belgacem MN, Gandini A. Monomers, polymers and composites from renewable resources. 1st ed. London: Elsevier; 2008. p. 530
Jayakumar R, Prabaharan M, Sudheesh KPT, et al. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011;29:322–37.
Jongrittiporn S, Kungsuwan A, Rakshit SK. A study on the preservation of fishballs using chitosan. in European Conference on Advanced Technology for Safe and High Quality Foods-EUROCAFT, Berlin, 2001.
Bottomley KMK, Bradshaw D, Nixon JS. Metalloproteinases as targets for anti-Inflammatory drugs. 1st ed. Basel: Birkhauser; 1999. p. 207
Muzzarelli R, Tarsi R, Filippini O, et al. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother. 1990;34:2019–23.
Tomihata K, Ikada Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials. 1997;18:567–73.
Liu XF, Guan YL, Yang DZ, et al. Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci. 2001;79(7):1324–35.
Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C. 2015;48:651–62.
García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–21.
Guo W, Guo Q, Zhang S, Li J. Manufacturing of artificial dura mater with chitosan polylactic acid. Chin J Clin Rehabil. 2005;9:24–25.
Sandoval-Sanchez JH, Ramos-Zuniga R, de Anda SL, et al. A new bilayer chitosan scaffolding as a dural substitute: experimental evaluation. World Neurosurg. 2012;77(3–4):577–82.
Kim H, Tator CH, Shoichet MS. Chitosan implants in the rat spinal cord: biocompatibility and biodegradation. J Biomed Mater Res. 2011;97(4):395–404.
Alleyne CH Jr, Barrow DL. Immune response in hosts with cadaveric dural grafts. Report of two cases. J Neurosurg. 1994;81:610–3.
Esposito F, Grimod G, Cavallo LM, et al. Collagen-only biomatrix as dural substitute: What happened after a 5-year observational follow-up study. Clin Neurol Neurosurg. 2013;115:1735–7.
Lang CJG, Heckmann JG, Neundorfer B. Creutzfeldt–Jakob disease via dural and corneal transplants. J Neurol Sci. 1998;160:128–39.
Baharuddin A, Go BT, Firdaus MNAR, et al. Bovine pericardium for dural graft: clinical results in 22 patients. Clin Neurol Neurosurg. 2002;104:342–4.
Mello LR, Feltrin LT, Fontesneto PT, Ferraz FA. Duraplasty with biosynthetic cellulose: an experimental study. J Neurosurg. 1997;86:143–50.
Vakis A, Koutentakis D, Karabetsos D, Kalostos G. Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2006;108:798–802.
Sabatino G, Pepa GMD, Bianchi F, et al. Autologous dural substitutes: a prospective study. Clin Neurol Neurosurg. 2014;116:20–3.
McCall TD, Fults DW, Schmidt RH. Use of resorbable collagen dural substitutes in the presence of cranial and spinal infections—report of three cases. Surg Neurol. 2008;70:92–7.
Sekhar LN, Mai JC. Dural repair after craniotomy and the use of dural substitutes and dural sealants. World Neurosurg. 2011;79(3–4):440–2.
von Wild KRH. Examination of the safety and efficacy of an absorbable dura mater substitute (Dura Patch®) in normal applications in neurosurgery. Surg Neurol. 1999;52:418–25.
Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90.
Esposito F, Cappabianca P, Fusco M. Collagen-only biomatrix as a novel dural substitute examination of the efficacy, safety and outcome: clinical experience on a series of 208 patients. Clin Neurol Neurosurg. 2008;110:343–351.
Timhadjelt L, Serier A, Belgacem MN, et al. Elaboration of cellulose based nanobiocomposite: effect of cellulose nanocrystals surface treatment and interface “melting”. Ind Crops Prod. 2015;72:7–15.
Yang TL. Chitin-based materials in tissue engineering: applications in soft tissue and epithelial organ. Int J Mol Sci. 2011;12(3):1936–1963.
Acknowledgment
The team of authors thank for Robert Owen, PhD student at the University of Sheffield, INSIGNEO Institute for silico Medicine for help with the cell culture experiments. M. Pogorielov was funded by Ukrainian Ministry of Education and Science for research visit to The University of Sheffield (2014 yr.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pogorielov, M., Kravtsova, A., Reilly, G. et al. Experimental evaluation of new chitin–chitosan graft for duraplasty. J Mater Sci: Mater Med 28, 34 (2017). https://doi.org/10.1007/s10856-017-5845-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5845-3