Abstract
Background and Aims
Hyperinsulinemia and insulin resistance are hallmark features of nonalcoholic fatty liver disease and steatohepatitis (NASH). It remains unclear whether and how insulin contributes to the development of fibrosis in NASH. In this study, we explored insulin signaling in the regulation of hepatic stellate cell (HSC) activation and the progression of NASH-fibrosis.
Methods
Phosphorylation of Akt and p70S6K were examined in primary HSC and in a rat model of NASH-fibrosis induced by high-fat and high-cholesterol diet for 24 weeks. HSC activation was analyzed for the changes in cell morphology, intracellular lipid droplets, expression of α-SMA and cell proliferation. The serum markers and histology for NASH-fibrosis were also characterized in animals.
Results
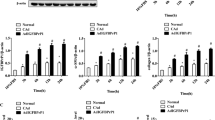
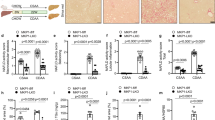
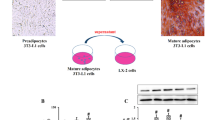
Insulin enhanced the expression of smooth muscle actin-α in quiescent but not in activated HSC in culture. Insulin-mediated activation of the PI3K/Akt-p70S6K pathway was involved in the regulation of profibrogenic effects of insulin. Although insulin did not stimulate HSC proliferation directly, the insulin-PI3K/Akt-p70S6K pathway was necessary for serum-enhanced cell proliferation during initial HSC activation. In a rat model of NASH-fibrosis induced by high-fat and high-cholesterol diet, hyperinsulinemia is associated with the activation of p70S6K and enhanced fibrosis.
Conclusion
The insulin-PI3K/Akt-p70S6K pathway plays an important role in the early activation of HSC. The profibrogenic effect of insulin is dependent on the activation stage of HSC. Dysregulation of the insulin pathway likely correlates with the development of fibrosis in NASH, suggesting a potentially novel antifibrotic target of inhibiting insulin signaling in HSC.






Similar content being viewed by others
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- HSC:
-
Hepatic stellate cells
- ECM:
-
Extracellular matrix
- α-SMA:
-
Smooth muscle actin-α
- NAS:
-
NAFLD activity score
- PI3K:
-
Phosphatidylinositol 3-kinase
- TGFβ:
-
Transforming growth factor β
- PDGF:
-
Platelet-derived growth factor
- Akt:
-
Protein kinase B
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-regulated kinase
- IR:
-
Insulin receptor
- IRS:
-
Insulin receptor substrate
- mTOR:
-
Mammalian target of rapamycin
- HFC diet:
-
High-fat and high-cholesterol diet
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FCS:
-
Fetal calf serum
- PBS:
-
Phosphate-buffered saline
- DMSO:
-
Dimethyl sulfoxide
- ECL:
-
Enhanced chemiluminescence
- H&E:
-
Hematoxylin and eosin
- BrdU:
-
5-Bromo-2′-deoxyuridine
- qPCR:
-
Quantitative polymerase chain reaction
- Col1A1:
-
α1 chain of type 1 collagen
- PPIA:
-
Peptidylprolyl isomerase A
- SEM:
-
Standard error of mean
References
Harrison SA, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis. 2004;8:861–879. (ix).
Neuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–198.
Brunt EM, Neuschwander-Tetri BA, Oliver D, Wehmeier KR, Bacon BR. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–1082.
Mehal WZ, Schuppan D. Antifibrotic therapies in the liver. Semin Liver Dis. 2015;35:184–198.
Wang P, Koyama Y, Liu X, et al. Promising therapy candidates for liver fibrosis. Front Physiol. 2016;7:47.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218.
Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172.
Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548.
Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416.
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci J Virtual Libr. 2002;7:d793–d807.
Yoshida K, Matsuzaki K. Differential regulation of TGF-beta/Smad signaling in hepatic stellate cells between acute and chronic liver injuries. Front Physiol. 2012;3:53.
Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563–1569.
Britton RS, Bacon BR. Intracellular signaling pathways in stellate cell activation. Alcohol Clin Exp Res. 1999;23:922–925.
Bridle KR, Li L, O’Neill R, Britton RS, Bacon BR. Coordinate activation of intracellular signaling pathways by insulin-like growth factor-1 and platelet-derived growth factor in rat hepatic stellate cells. J Lab Clin Med. 2006;147:234–241.
Liu C, Li J, Xiang X, et al. PDGF receptor-alpha promotes TGF-beta signaling in hepatic stellate cells via transcriptional and posttranscriptional regulation of TGF-beta receptors. Am J Physiol Gastrointest Liver Physiol. 2014;307:G749–G759.
van der Poorten D, George J. Disease-specific mechanisms of fibrosis: hepatitis C virus and nonalcoholic steatohepatitis. Clin Liver Dis. 2008;12:805–824. (ix).
Rombouts K, Marra F. Molecular mechanisms of hepatic fibrosis in non-alcoholic steatohepatitis. Dig Dis. 2010;28:229–235.
Bian Z, Ma X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol. 2012;3:248.
Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35:132–145.
Tomita K, Teratani T, Suzuki T, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–169.
Wallace MC, Friedman SL, Mann DA. Emerging and disease-specific mechanisms of hepatic stellate cell activation. Semin Liver Dis. 2015;35:107–118.
Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol. 2007;47:142–156.
Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761.
Fartoux L, Poujol-Robert A, Guechot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008.
Ota T, Takamura T, Kurita S, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–293.
Bugianesi E, Manzini P, D’Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187.
Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751.
Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Invest. 2009;89:1397–1409.
Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744.
Zhang F, Zhang Z, Kong D, et al. Tetramethylpyrazine reduces glucose and insulin-induced activation of hepatic stellate cells by inhibiting insulin receptor-mediated PI3K/AKT and ERK pathways. Mol Cell Endocrinol. 2014;382:197–204.
Yoneda A, Sakai-Sawada K, Niitsu Y, Tamura Y. Vitamin A and insulin are required for the maintenance of hepatic stellate cell quiescence. Exp Cell Res. 2016;341:8–17.
Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96.
Gabele E, Reif S, Tsukada S, et al. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374–13382.
Farrar CT, DePeralta DK, Day H, et al. 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J Hepatol. 2015;63:689–696.
Kim YJ, Lee ES, Kim SH, et al. Inhibitory effects of rapamycin on the different stages of hepatic fibrosis. World J Gastroenterol. 2014;20:7452–7460.
Wang W, Yan J, Wang H, et al. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PloS One. 2014;9:e83908.
Patsenker E, Schneider V, Ledermann M, et al. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55:388–398.
Bridle KR, Popa C, Morgan ML, et al. Rapamycin inhibits hepatic fibrosis in rats by attenuating multiple profibrogenic pathways. Liver Transpl. 2009;15:1315–1324.
Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786–796.
Biecker E, De Gottardi A, Neef M, et al. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313:952–961.
Zhu J, Wu J, Frizell E, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117:1198–1204.
Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi:10.1007/s10620-009-0815-3.
Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PloS One. 2013;8:e76538.
Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207–218.
Tura A, Pacini G, Kautzky-Willer A, Ludvik B, Prager R, Thomaseth K. Basal and dynamic proinsulin-insulin relationship to assess beta-cell function during OGTT in metabolic disorders. Am J Physiol Endocrinol Metab. 2003;285:E155–E162.
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321.
Kubrusly MS, Correa-Giannella ML, Bellodi-Privato M, et al. A role for mammalian target of rapamycin (mTOR) pathway in non alcoholic steatohepatitis related-cirrhosis. Histol Histopathol. 2010;25:1123–1131.
Asai A, Chou PM, Bu HF, et al. Dissociation of hepatic insulin resistance from susceptibility of nonalcoholic fatty liver disease induced by a high-fat and high-carbohydrate diet in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G496–G504.
Mei S, Yang X, Guo H, et al. A small amount of dietary carbohydrate can promote the HFD-induced insulin resistance to a maximal level. PloS One. 2014;9:e100875.
Ferreira DM, Castro RE, Machado MV, et al. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54:1788–1798.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274.
Adachi M, Osawa Y, Uchinami H, Kitamura T, Accili D, Brenner DA. The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells. Gastroenterology. 2007;132:1434–1446.
Xiao J, Ho CT, Liong EC, et al. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53:187–199.
Valenti L, Rametta R, Dongiovanni P, et al. Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes. 2008;57:1355–1362.
Asgharpour A, Cazanave SC, Pacana T, et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol. 2016;65:579–588.
Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924.
He L, Gubbins J, Peng Z, et al. Activation of hepatic stellate cell in Pten null liver injury model. Fibrogenesis Tissue Repair. 2016;9:8.
Acknowledgments
The study was supported by the Institutional Research Fund from VA Loma Linda Healthcare System/Loma Linda Veterans Association for Research and Education, and Saint Louis University Liver Center. The authors thank Drs. Subburaman Mohan, Donna Strong, Hidekazu Tsukamoto, David A. Brenner and Neil Kaplowitz for their helpful discussions on the project. We are grateful to Rosemary O’Neill, Vanita Talkad, Wei Zhao, Lucy Zhao, Wei-fen Hsu and Subhashri Das for technical assistances.
Financial support
VA Loma Linda Healthcare System/Loma Linda Veterans Association for Research and Education, Saint Louis University Liver Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest related to the study.
Rights and permissions
About this article
Cite this article
Cai, C.X., Buddha, H., Castelino-Prabhu, S. et al. Activation of Insulin-PI3K/Akt-p70S6K Pathway in Hepatic Stellate Cells Contributes to Fibrosis in Nonalcoholic Steatohepatitis. Dig Dis Sci 62, 968–978 (2017). https://doi.org/10.1007/s10620-017-4470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4470-9