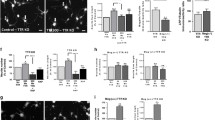
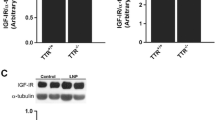
Abstract
The peptide preparation semax has been effectively used for therapy of ischemic stroke. However, the mechanisms of its action are insufficiently understood and actively studied. The full-genome analysis of the transcriptome carried out in our recent work demonstrated that under conditions of focal ischemia of rat brain the semax modified the profile of the transcription activity of many genes. In this case, the difference in the transcription levels of the gene encoding the protein transthyretin (Ttr) expression in rats under the pathological conditions of ischemia and in the presence of semax was very high. A high similarity between the effects of Ttr and coupled molecular systems with the semax effects in ischemic stroke allowed us to suggest that the neuroprotection mechanisms of semax (and, possibly, of other neuroprotection mechanisms of semax) may be mediated by Ttr. In this review, we discuss the role of Ttr in the central nervous system and its possible role in the neuroprotection mechanism of semax.
Similar content being viewed by others
References
Silachev, D.N., Shram, S.I., Shakova, F.M., Romanova, G.A., and Myasoedov, N.F., Formation of spatial memory in rats with ischemic lesions to the prefron-tal cortex; effects of a synthetic analog of ACTH (4-7), Neurosci. Behav. Physiol., 2009, vol. 39, pp. 749–756.
Romanova, G.A., Silachev, D.N., Shakova, F.M., Kvashennikova, Y.N., Viktorov, I.V., Shram, S.I., et al., Neuroprotective and antiamnesic effects of Semax during experimental ischemic infarction of the cerebral cortex, Bull. Exp. Biol. Med., 2006, vol. 142, pp. 663–666.
Kaplan, A.Ya., Koshelev, V.B., Nezavibat’ko, V.N., and Ashmarin, I.P., The way to increase an organism resistance against hypoxia with the help of neuropeptide drug semax, Fiziol. Chel., 1992, vol. 18, no. 9, pp. 104–107.
Gusev, E.I., Skvortsova, V.I., Miasoedov, N.F., Nezavibat’ko, V.N., Zhuravleva, E.I., and Vanichkin, A.V., Semax efficiency in acute period of hemispheric ischemic stroke, Zh. Nevrol. Psikhiatr. im. S.S. Korsakova, 1997, vol. 97, pp. 26–34.
Miasoedov, N.F., Skvortsova, V.I., Nasonov, E.L., Zhuravleva, E.I., Grivennikov, I.A., and Arsen’eva, E.L., et al., Semax neuroprotective effect in acute period of ischemic stroke, Zh. Nevrol. Psikhiatr. im. S.S. Korsakova, 1999, vol. 5, pp. 15–19.
Alekseeva, G.V., Bottaeva, N.A., and Gorshkova, V.V., Semax application in patients with post-hypoxic brain pathology for long-term period, Anesteziol. Reanimatol., 1999, vol. 1, pp. 40–43.
Medvedeva, E.V., Dmitrieva, V.G., Povarova, O.V., Limborska, S.A., Skvortsova, V.I., Myasoedov, N.F., et al., The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: Genome-wide transcriptional analysis, BMC Genomics, 2014, vol. 15, p. 228.
Medvedeva, E.V., Limborskaya, S.A., Myasoedov, N.F., and Dergunova, L.V., Semax positive effect onto TTR gene expression in the model of rats’ brain focal ischemia, Materialy 7-go Rossiiskogo simpoziuma “Belki i peptidy” (Proc. 7th Russian Symposium “Proteins and Peptides”), Novosibirsk, 2015, p. 367.
Nomenclature Committee of IUB (NC-IUB) IUBIUPAC Joint Commission on Biochemical Nomenclature (JCBN). Newsletter. 1981, J. Biol. Chem., 1981, vol. 256, pp. 12–14.
Ando, Y., Transthyretin: It’s miracle function and pathogenesis, Rinsho Byori Jpn. J. Clin. Pathol., 2009, vol. 57, pp. 228–235.
Richardson, S.J., Evolutionary changes to transthyretin: Evolution of transthyretin biosynthesis, FEBS J., 2009, vol. 276, pp. 5342–5356.
Alshehri, B., D’Souza, D.G., Lee, J.Y., Petratos, S., and Richardson, S.J., The diversity of mechanisms influenced by transthyretin in neurobiology: Development, disease and endocrine disruption, J. Neuroendocrinol., 2015, vol. 27, pp. 303–323.
Herbert, J., Wilcox, J.N., Pham, K.T., Fremeau, R.T., Zeviani, M., Dwork, A., et al., Transthyretin: A choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award, Neurology, 1986, vol. 36, pp. 900–911.
Fleming, C.E., Nunes, A.F., and Sousa, M.M., Transthyretin: More than meets the eye, Prog. Neurobiol., 2009, vol. 89, pp. 266–276.
Brouillette, J. and Quirion, R., Transthyretin: A key gene involved in the maintenance of memory capacities during aging, Neurobiol. Aging, 2008, vol. 29, pp. 1721–1732.
Fleming, C.E., Saraiva, M.J., and Sousa, M.M., Transthyretin enhances nerve regeneration, J. Neurochem., 2007, vol. 103, pp. 831–839.
Sousa, J.C., Grandela, C., Fernández-Ruiz, J., de Miguel, R., de Sousa, L., Magalhães, A.I., et al., Transthyretin is involved in depression-like behavior and exploratory activity, J. Neurochem., 2004, vol. 88, pp. 1052–1058.
Gao, C., Zhang, B., Zhang, W., Pu, S., Yin, J., and Gao, Q., Serum prealbumin (transthyretin) predict good outcome in young patients with cerebral infarction, Clin. Exp. Med., 2011, vol. 11, pp. 49–54.
Santos, S.D., Lambertsen, K.L., Clausen, B.H., Akinc, A., Alvarez, R., Finsen, B., et al., CSF transthyretin neuroprotection in a mouse model of brain ischemia, J. Neurochem., 2010, vol. 115, pp. 1434–1444.
Richardson, S.J., Wijayagunaratne, R.C., D’Souza, D.G., Darras, V.M., and Van Herck S.L.J., Transport of thyroid hormones via the choroid plexus into the brain: The roles of transthyretin and thyroid hormone transmembrane transporters, Front. Neurosci., 2015, vol. 9, p. 66.
Schroeder, A.C. and Privalsky, M.L., Thyroid hormones, T3 and T4, in the brain, Front. Endocrinol. (Rome, Italy), 2014, vol. 5, p. 40.
Bauer, M., Goetz, T., Glenn, T., and Whybrow, P.C., The thyroid–brain interaction in thyroid disorders and mood disorders, J. Neuroendocrinol., 2008, vol. 20, pp. 1101–1114.
Ahmed, O.M., El-Gareib, A.W., El-Bakry, A.M., Abd El-Tawab, S.M., Ahmed, R.G., Thyroid hormones states and brain development interactions, Int. J. Dev. Neurosci., 2008, vol. 26, pp. 147–209.
Remaud, S., Gothié, J.-D., Morvan-Dubois, G., and Demeneix, B.A., Thyroid hormone signaling and adult neurogenesis in mammals, Front. Endocrinol. (Rome, Italy), 2014, vol. 5, p. 62.
Naylor, H.M. and Newcomer, M.E., The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP, Biochemistry, 1999, vol. 38, pp. 2647–2653.
Goodman, A.B. and Pardee, A.B., Evidence for defective retinoid transport and function in late onset Alzheimer’s disease, Proc. Natl. Acad. Sci. U.S.A., 2003, vol. 100, pp. 2901–2905.
LaMantia, A.S., Forebrain induction,retinoic acid,and vulnerability to schizophrenia: Insights from molecular and genetic analysis in developing mice, Biol. Psychiatry, 1999, vol. 46, pp. 19–30.
Lane, M.A. and Bailey, S.J., Role of retinoid signaling in the adult brain, Prog. Neurobiol., 2005, vol. 75, pp. 275–293.
Etchamendy, N., Enderlin, V., Marighetto, A., Pallet, V., Higueret, P., and Jaffard, R., Vitamin A deficiency and relational memory deficit in adult mice: Relationships with changes in brain retinoid signaling, Behav. Brain Res., 2003, vol. 145, pp. 37–49.
Olson, C.R. and Mello, C.V., Significance of vitamin A to brain function, behavior and learning, Mol. Nutr. Food Res., 2010, vol. 54, pp. 489–495.
Chakrabarti, M., McDonald, A.J., Will Reed, J., Moss, M.A., Das, B.C., and Ray, S.K., Molecular signaling mechanisms of natural and synthetic retinoids for inhibition of pathogenesis in Alzheimer’s disease, J. Alzheimer’s Dis., 2015, vol. 50, pp. 335–352.
Maden, M. and Hind, M., Retinoic acid, a regeneration-inducing molecule, Dev. Dyn., 2003, vol. 226, pp. 237–244.
Sato, Y., Meller, R., Yang, T., Taki, W., and Simon, R.P., Stereo-selective neuroprotection against stroke with vitamin A derivatives, Brain Res., 2008, vol. 1241, pp. 188–192.
Harvey, B.K., Shen, H., Chen, G.-J., Yoshida, Y., and Wang, Y., Midkine and retinoic acid reduce cerebral infarction induced by middle cerebral artery ligation in rats, Neurosci. Lett., 2004, vol. 369, pp. 138–141.
Shen, H., Luo, Y., Kuo, C.-C., Deng, X., Chang, C.-F., Harvey, B.K., et al., 9-Cis-retinoic acid reduces ischemic brain injury in rodents via bone morphogenetic protein, J. Neurosci. Res., 2009, vol. 87, pp. 545–555.
Kitamura, M., Ishikawa, Y., Moreno-Manzano, V., Xu, Q., Konta, T., Lucio-Cazana, J., et al., Intervention by retinoic acid in oxidative stress-induced apoptosis, Nephrol., Dial., Transplant., 2002, vol. 17, no. 9, pp. 84–87.
Costa, R.H., Van Dyke, T.A., Yan, C., Kuo, F., and Darnell, J.E., Similarities in transthyretin gene expression and differences in transcription factors: Liver and yolk sac compared to choroid plexus, Proc. Natl. Acad. Sci. U.S.A., 1990, vol. 87, pp. 6589–6593.
Jaeger, A.M., Makley, L.N., Gestwicki, J.E., and Thiele, D.J., Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity, J. Biol. Chem., 2014, vol. 289, pp. 30459–30469.
Wang, X., Cattaneo, F., Ryno, L., Hulleman, J., Reixach, N., and Buxbaum, J.N., The systemic amyloid precursor transthyretin (TTR) behaves as a neuronal stress protein regulated by HSF1 in SH-SY5Y human neuroblastoma cells and APP23 Alzheimer’s disease model mice, J. Neurosci., 2014, vol. 34, pp. 7253–7265.
Ivanova, N.E., The results of the semax application under cognitive impairments in acute ischemic stroke and chronic cerebral ischemia, Eff. Farmakoter., 2012, no. 2, pp. 8–13.
Genovese, T., Impellizzeri, D., Ahmad, A., Cornelius, C., Campolo, M., Cuzzocrea, S., et al., Post-ischemic thyroid hormone treatment in a rat model of acute stroke, Brain Res., 2013, vol. 1513, pp. 92–102.
Storozhevykh, T.P., Tukhbatova, G.R., Senilova, Ya.E., Pinelis, V.G., Andreeva, L.A., and Myasoyedov, N.F., Effects of semax and its Pro-Gly-Pro fragment on calcium homeostasis of neurons and their survival under conditions of glutamate toxicity, Bull. Exp. Biol. Med., 2007, vol. 143, no. 5, pp. 601–604.
Lee, E.J., Bhat, A.R., Kamli, M.R., Pokharel, S., Chun, T., Lee, Y.-H., et al., Transthyretin is a key regulator of myoblast differentiation, PLoS One, 2013, vol. 8, no. 5, p. e63627.
de Vito, P., Balducci, V., Leone, S., Percario, Z., Mangino, G., Davis, P.J., et al., Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players, Steroids, 2012, vol. 77, pp. 988–995.
Inouye, S., Izu, H., Takaki, E., Suzuki, H., Shirai, M., Yokota, Y., et al., Impaired IgG production in mice deficient for heat shock transcription factor 1, J. Biol. Chem., 2004, vol. 279, pp. 38701–38709.
Stavchansky, V.V., Yuzhakov, V.V., Botsina, A.Y., Skvortsova, V.I., Bondurko, L.N., Tsyganova, M.G., et al., The effect of Semax and its C-end peptide PGP on the morphology and proliferative activity of rat brain cells during experimental ischemia: A pilot study, J. Mol. Neurosci., 2011, vol. 45, pp. 177–185.
Richardson, S.J., Lemkine, G.F., Alfama, G., Hassani, Z., and Demeneix, B.A., Cell division and apoptosis in the adult neural stem cell niche are differentially affected in transthyretin null mice, Neurosci. Lett., 2007, vol. 421, pp. 234–238.
Lemkine, G.F., Raj, A., Alfama, G., Turque, N., Hassani, Z., Alegria-Prévot, O., et al., Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor, FASEB J., 2005, vol. 19, no. 7, pp. 863–865.
Fernandez, M., Pirondi, S., Manservigi, M., Giardino, L., and Calzà, L., Thyroid hormone participates in the regulation of neural stem cells and oligodendrocyte precursor cells in the central nervous system of adult rat, Eur. J. Neurosci., 2004, vol. 20, pp. 2059–2070.
Dirks, P., Tieding, S., Schneider, I., Mey, J., and Weiler, R., Characterization of retinoic acid neuromodulation in the carp retina, J. Neurosci. Res., 2004, vol. 78, no. 2, pp. 177–185.
Zhang, D.Q. and McMahon, D.G., Direct gating by retinoic acid of retinal electrical synapses, Proc. Natl. Acad. Sci. U.S.A., 2000, vol. 97, no. 26, pp. 14754–14759.
Alekseev, I.B., Lomakina, O.E., Shinalieva, O.N., and Alekseeva, G.N., The efficiency of Semax 0.1% as a neuroprotective therapy in patients with glaucoma, Glaukoma, 2012, no. 1, pp. 48–52.
Eremin, K.O., Kudrin, V.S., Saransaari, P., Oja, S.S., Grivennikov, I.A., Myasoedov, N.F., et al., Semax,an ACTH (4-10) analogue with nootropic properties, activates dopaminergic and serotoninergic brain systems in rodents, Neurochem. Res., 2005, vol. 30, no. 12, pp. 1493–1500.
Tatum, M.C., Ooi, F.K., Chikka, M.R., Chauve, L., Martinez-Velazquez, L.A., Steinbusch, H.W.M., et al., Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase, Curr. Biol., 2015, vol. 25, no. 2, pp. 163–174.
Chiang, M.-C., Chen, H.-M., Lai, H.-L., Chen, H.-W., Chou, S.-Y., Chen, C.-M., et al., The A2A adenosine receptor rescues the urea cycle deficiency of Huntington’s disease by enhancing the activity of the ubiquitinproteasome system, Hum. Mol. Genet., 2009, vol. 18, no. 16, pp. 2929–2942.
Doepner, R.F.G., Geigerseder, C., Frungieri, M.B., Gonzalez-Calvar, S.I., Calandra, R.S., Raemsch, R., et al., Insights into GABA receptor signaling in TM3 Leydig cells, Neuroendocrinology, 2005, vol. 81, no. 6, pp. 381–390.
Bromberg, Z., Goloubinoff, P., Saidi, Y., and Weiss, Y.G., The membrane-associated transient receptor potential vanilloid channel is the central heat shock receptor controlling the cellular heat shock response in epithelial cells, PLoS One, 2013, vol. 8, no. 2, p. e57149.
Bezuglov, V.V., Akimov, M.G., Gretskaya, N.M., Surin, A.M., Pinelis, V.G., Shram, S.I., et al., The study of the neurotropic peptides role in cell responses regulation, in Horizons in Neuroscience Research, Costa, A. and Villalba, E., Eds., New York: Nova Sci. Publ., 2015, chap. 10, pp. 151–170.
V’yunova, T.V. and Andreeva, L.A., Peptide interaction with receptor systems of cells, Materialy 6-oi mezhdunarodnoi konferentsii “Biologicheskie osnovy individual’noi chuvstvitel’nosti k psikhotropnym sredstvam (Proc. 6th Int. Conference “Biological Foundations of Individual Sensitivity to Psychotropic Drugs”), Moscow, Nov. 9–13, 2015; Eksp. Klin. Farmakol., 2015, vol. 78, no. 999, p. 17.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.V. Vyunova, E.V. Medvedeva, L.A. Andreeva, L.V. Dergunova, S.A. Limborska, N.F. Myasoedov, 2016, published in Molekulyarnaya Genetika, Mikrobiologiya i Virusologiya, 2016, No. 3, pp. 104–109.
Genes, human and animal proteins in the review are used in accordance with the international nomenclature in NCBI databases. Transthyretin protein of humans and animals is given as TTR and Ttr, respectively. Genes are given in italics for humans and animals as TTR and Ttr.
About this article
Cite this article
Vyunova, T.V., Medvedeva, E.V., Andreeva, L.A. et al. A possible role of transthyretin in the biological mechanism of regulatory peptide neuroprotection. Mol. Genet. Microbiol. Virol. 31, 143–148 (2016). https://doi.org/10.3103/S0891416816030101
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416816030101