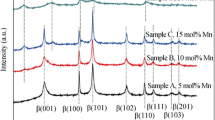
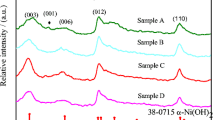
Abstract
Nickel hydroxides with hierarchical micro-nano structures are prepared by a facile homogeneous precipitation method with different nickel salts (Ni(NO3)2·6H2O, NiCl2·6H2O, and NiSO4·6H2O) as raw materials. The effect of nickel sources on the microstructure and lithium storage performance of the nickel hydroxides is studied. It is found that all the three prepared samples are α-nickel hydroxide. The nickel hydroxides synthesized with Ni(NO3)2·6H2 or NiCl2·6H2O show a similar particle size of 20–30 μm and are composed of very thin nano-sheets, while the nickel hydroxide synthesized with Ni(SO4)2·6H2O shows a larger particle size (30–50 μm) and consists of very thin nano-walls. When applied as anode materials for lithium-ion batteries (LIBs), the nickel hydroxide synthesized with NiSO4·6H2O exhibits the highest discharge capacity, but its cyclic stability is very poor. The nickel hydroxides synthesized with NiCl2·6H2O exhibit higher discharge capacity than the nickel hydroxides synthesized with Ni(NO3)2·6H2O, and both of them show much improved cyclic stability and rate capability as compared to the nickel hydroxide synthesized with Ni(SO4)2·6H2O. Moreover, pseudocapacitive behavior makes a great contribution to the electrochemical energy storage of the three samples. The discrepancies of lithium storage performance of the three samples are analyzed by ex-situ XRD, FT-IR, electrochemical impedance spectroscopy (EIS), and cyclic voltammetry (CV) tests.








Similar content being viewed by others

References
Park OK, Cho Y, Lee S, Yoo H-C, Song H-K, Cho J (2011) Who will drive electric vehicles, olivine or spinel? Energ Environ Sci 4:1621–1633
Jaguemont J, Boulon L, Dube Y (2016) A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl Energy 164:99–114
Yi T-F, Yang S-Y, Xie Y (2015) Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A 3:5750–5777
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energ Environ Sci 4:3243–3262
Pan L, Zhu X-D, Xie X-M, Liu Y-T (2015) Smart hybridization of TiO2 nanorods and Fe3O4 nanoparticles with pristine graphene nanosheets: hierarchically nanoengineered ternary heterostructures for high-rate lithium storage. Adv Funct Mater 25:3341–3350
Yu SH, Lee SH, Lee DJ, Sung YE, Hyeon T (2016) Conversion reaction‐based oxide nanomaterials for lithium ion battery anodes. Small 12:2146–2172
Zhao Y, Li XF, Yan B, Xiong DB, Li DJ, Lawes S, Sun XL (2016) Recent developments and understanding of novel mixed transition-metal oxides as anodes in lithium ion batteries. Adv Energy Mater 6. doi:10.1002/aenm.201502175
Zhang JJ, Yu AS (2015) Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries. Sci Bull 60:823–838
Pan L, Wang K-X, Zhu X-D, Xie X-M, Liu Y-T (2015) Hierarchical assembly of SnO2 nanowires on MnO2 nanosheets: a novel 1/2D hybrid architecture for high-capacity, reversible lithium storage. J Mater Chem A 3:6477–6483
Feng LD, Zhu YF, Ding HY, Ni CY (2014) Recent progress in nickel based materials for high performance pseudocapacitor electrodes. J Power Sources 267:430–444
Gao XP, Yang HX (2010) Multi-electron reaction materials for high energy density batteries. Energ Environ Sci 3:174–189
Hall DS, Lockwood DJ, Bock C, MacDougall BR (2015) Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proc R Soc A The Royal Society 471:20140792
Li B, Cao H, Shao J, Zheng H, Lu Y, Yin J, Qu M (2011) Improved performances of β-Ni (OH)2@ reduced-graphene-oxide in Ni-MH and Li-ion batteries. Chem Commun 47:3159–3161
Buller S, Strunk J (2016) Nanostructure in energy conversion. J Energy Chem 25:171–190
Bruce PG, Scrosati B, Tarascon JM (2008) Nanomaterials for rechargeable lithium batteries. Angew Chem Int Edit 47:2930–2946
Wu S, Xu R, Lu M, Ge R, Iocozzia J, Han C, Jiang B, Lin Z (2015) Graphene‐containing nanomaterials for lithium‐ion batteries. Adv Energy Mater 5:1500400–1500439
Ni S, Lv X, Li T, Yang X, Zhang L (2013) The investigation of Ni(OH)2/Ni as anodes for high performance Li-ion batteries. J Mater Chem A 1:1544–1547
Tian J, Xing Z, Chu Q, Liu Q, Asiri AM, Qusti AH, Al-Youbi AO, Sun X (2013) PH-driven dissolution–precipitation: a novel route toward ultrathin Ni(OH)2 nanosheets array on nickel foam as binder-free anode for Li-ion batteries with ultrahigh capacity. CrystEngComm 15:8300–8305
Caballero A, Hernan L, Morales J, Cabanas-Polo S, Ferrari B, Sanchez-Herencia AJ, Canales-Vazquez J (2013) Electrochemical properties of ultrasonically prepared Ni(OH)2 nanosheets in lithium cells. J Power Sources 238:366–371
Tian L-L, Wei X-Y, Zhuang Q-C, Wu C, Xie R-L, Zong Z-M, Cui Y-L, Sun S-G (2013) Chiseled nickel hydroxide nanoplates growth on graphene sheets for lithium ion batteries. Nano 8:1350068
Zhu X, Zhong Y, Zhai H, Yan Z, Li D (2014) Nanoflake nickel hydroxide and reduced graphene oxide composite as anode materials for high capacity lithium ion batteries. Electrochim Acta 132:364–369
Min S, Zhao C, Ju P, Zhou T, Gao H, Zheng Y, Wang H, Chen G, Qian X, Guo Z (2016) Facile synthesis of nickel-foam-based nano-architectural composites as binder-free anodes for high capacity Li-ion batteries. J Power Sources 304:311–318
Hu W-K, Noréus D (2003) Alpha nickel hydroxides as lightweight nickel electrode materials for alkaline rechargeable cells. Chem Mater 15:974–978
Khan AI, O’Hare D (2002) Intercalation chemistry of layered double hydroxides: recent developments and applications. J Mater Chem 12:3191–3198
Shangguan EB, Li J, Guo D, Guo LT, Nie MZ, Chang ZR, Yuan XZ, Wang HJ (2015) A comparative study of structural and electrochemical properties of high-density aluminum substituted α-nickel hydroxide containing different interlayer anions. J Power Sources 282:158–168
Li YW, Yao JH, Liu CJ, Zhao WM, Deng WX, Zhong SK (2010) Effect of interlayer anions on the electrochemical performance of Al-substituted α-type nickel hydroxide electrodes. Int J Hydrog Energy 35:2539–2545
Wu X, Du YL, An X, Xie XM (2014) Fabrication of NiFe layered double hydroxides using urea hydrolysis—Control of interlayer anion and investigation on their catalytic performance. Catal Commun 50:44–48
Hongo T, Wakasa H, Yamazaki A (2011) Synthesis and adsorption properties of nanosized Mg-Al layered double hydroxides with Cl−, NO3 − or SO4 2− as interlayer anion. Mater Sci-Poland 29:86–91
Kamath PV, Dixit M, Indira L, Shukla AK, Kumar VG, Munichandraiah N (1994) bilized α‐Ni(OH)2 as Electrode Material for Alkaline Secondary Cells. J Electrochem Soc 141:2956–2959
Shao M, Han J, Wei M, Evans DG, Duan X (2011) The synthesis of hierarchical Zn–Ti layered double hydroxide for efficient visible-light photocatalysis. Chem Eng J 168:519–524
Li YW, Zhu YX, Yao JH, Shang X, Yang JW (2014) Effects of liquid anions on the structure and electrochemical properties of synthesized Al-substituted α-Ni(OH)2. J Chem Eng Chin Univ 28:864–869
Ertaş FS, Kaş R, Ünal U, Birer Ö (2013) Sonochemical synthesis and electrochemical characterization of α-nickel hydroxide: precursor effects. J Solid State Electrochem 17:1455–1462
Li Y, Yao J, Zhu Y, Zou Z, Wang H (2012) Synthesis and electrochemical performance of mixed phase α/β nickel hydroxide. J Power Sources 203:177–183
Ren Y, Gao L (2010) From three-dimensional flower-like α-Ni(OH)2 nanostructures to hierarchical porous NiO nanoflowers: microwave-assisted fabrication and supercapacitor properties. J Am Ceram Soc 93:3560–3564
Jobbágy M, Soler-IIIia GJAA, Regazzoni AE, Blesa MA (1998) Synthesis of copper (II)-containing nickel (II) hydroxide particles as precursors of copper (II)-substituted nickel (II) oxides. Chem Mat 10:1632–1637
Li Y, Pan G, Xu W, Yao J, Zhang L (2016) Effect of Al substitution on the microstructure and lithium storage performance of nickel hydroxide. J Power Sources 307:114–121
Hu Z-A, Xie Y-L, Wang Y-X, Wu H-Y, Yang Y-Y, Zhang Z-Y (2009) Synthesis and electrochemical characterization of mesoporous CoxNi1−x layered double hydroxides as electrode materials for supercapacitors. Electrochim Acta 54:2737–2741
Faure C, Delmas C, Fouassier M (1991) Characterization of a turbostratic α-nickel hydroxide quantitatively obtained from an NiSO4 solution. J Power Sources 35:279–290
Nethravathi C, Ravishankar N, Shivakumara C, Rajamathi M (2007) Nanocomposites of α-hydroxides of nickel and cobalt by delamination and co-stacking: Enhanced stability of α-motifs in alkaline medium and electrochemical behaviour. J Power Sources 172:970–974
Kosova NV, Devyatkina ET, Kaichev VV (2007) Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition. J Power Sources 174:735–740
Pan T, Wang JM, Zhao YL, Chen H, Xiao HM, Zhang JQ (2003) Al-stabilized α-nickel hydroxide prepared by electrochemical impregnation. Mater Chem Phys 78:711–718
Zhu Y, Cao C (2015) Remarkable electrochemical lithium storage behaviour of two-dimensional ultrathin alpha-Ni(OH)2 nanosheets. RSC Adv 5:83757–83763
Ni SB, Li T, Yang XL (2012) Fabrication of NiO nanoflakes and its application in lithium ion battery. Mater Chem Phys 132:1108–1111
Ni SB, Lv XH, Ma JL, Yang XL, Zhang LL (2014) A novel electrochemical reconstruction in nickel oxide nanowalls on Ni foam and the fine electrochemical performance as anode for lithium ion batteries. J Power Sources 270:564–568
Wang X, Li X, Sun X, Li F, Liu Q, Wang Q, He D (2011) Nanostructured NiO electrode for high rate Li-ion batteries. J Mater Chem 21:3571
Wang C, Wang D, Wang Q, Chen H (2010) Fabrication and lithium storage performance of three-dimensional porous NiO as anode for lithium-ion battery. J Power Sources 195:7432–7437
Cabanas-Polo S, Gonzalez Z, Sanchez-Herencia AJ, Ferrari B, Caballero A, Hernan L, Morales J (2015) Cyclability of binder-free β-Ni(OH)2 anodes shaped by EPD for Li-ion batteries. J Eur Ceram Soc 35:573–584
Niu K-Y, Lin F, Fang L, Nordlund D, Tao R, Weng T-C, Doeff MM, Zheng H (2015) Structural and chemical evolution of amorphous nickel iron complex hydroxide upon lithiation/delithiation. Chem Mater 27:1583–1589
Wu T, Liang K (2016) Caterpillar structured Ni(OH)2@MnO2 core/shell nanocomposite arrays on nickel foam as high performance anode materials for lithium ion batteries. RSC Adv 6:15541–15548
Yang L, Li X, He S, Du G, Yu X, Liu J, Gao Q, Hu R, Zhu M (2016) Mesoporous Mo2C/N-doped Carbon Heteronanowires as High Performance Anode materials for Li-Ion Batteries. J Mater Chem A 4:10842–10849
Zhong S, Chen W, Wu L, Liu J (2012) A PEG-assisted rheological phase reaction synthesis of 5LiFePO4⋅Li3V2(PO4)3/C as cathode material for lithium ion cells. Ionics 18:523–527
Rammelt U, Reinhard G (1990) On the applicability of a constant phase element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim Acta 35:1045–1049
Devos O, Gabrielli C, Tribollet B (2006) Simultaneous EIS and in situ microscope observation on a partially blocked electrode application to scale electrodeposition. Electrochim Acta 51:1413–1422
Yamada Y, Sasaki T, Tatsuda N, Weingarth D, Yano K, Kötz R (2012) A novel model electrode for investigating ion transport inside pores in an electrical double-layer capacitor: Monodispered microporous starburst carbon spheres. Electrochim Acta 81:138–148
Zhang SS (2006) A review on electrolyte additives for lithium-ion batteries. J Power Sources 162:1379–1394
He Y-B, Ning F, Li B, Song Q-S, Lv W, Du H, Zhai D, Su F, Yang Q-H, Kang F (2012) Carbon coating to suppress the reduction decomposition of electrolyte on the Li4Ti5O12 electrode. J Power Sources 202:253–261
Yang CR, Wang YY, Wan CC (1998) Composition analysis of the passive film on the carbon electrode of a lithium-ion battery with an EC-based electrolyte. J Power Sources 72:66–70
Ota H, Kominato A, Chun W-J, Yasukawa E, Kasuya S (2003) Effect of cyclic phosphate additive in non-flammable electrolyte. J Power Sources 119–121:393–398
Ostrovskii D, Ronci F, Scrosati B, Jacobsson P (2001) A FTIR and Raman study of spontaneous reactions occurring at the LiNiyCo(1− y)O2 electrode/non-aqueous electrolyte interface. J Power Sources 94:183–188
Wang Z, Huang Y, Wang X, Jia D, Guo Z, Miao M (2013) Tetraethoxysilane as a new facilitative film-forming additive for the lithium-ion battery with LiMn2O4 cathode. Solid State Ionics 232:19–23
Chalasani D, Li J, Jackson NM, Payne M, Lucht BL (2012) Methylene ethylene carbonate: Novel additive to improve the high temperature performance of lithium ion batteries. J Power Sources 208:67–73
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7:1597–1614
Liu TC, Pell WG, Conway BE, Roberson SL (1998) Behavior of molybdenum nitrides as materials for electrochemical capacitors comparison with ruthenium oxide. J Electrochem Soc 145:1882–1888
Wang J, Polleux J, Lim J, Dunn B (2007) Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J Phys Chem C 111:14925–14931
Rauda IE, Augustyn V, Saldarriaga-Lopez LC, Chen X, Schelhas LT, Rubloff GW, Dunn B, Tolbert SH (2014) Nanostructured pseudocapacitors based on atomic layer deposition of V2O5 onto conductive nanocrystal‐based mesoporous ITO scaffolds. Adv Funct Mater 24:6717–6728
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21263003, 51664012, 51464009, 51204061), Guangxi Natural Science Foundation of China (2015GXNSFGA139006, 2014GXNSFBA118238), and Key Laboratory of Renewable Energy, Chinese Academy of Sciences (No. y507k61001).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Y., Xu, W., Xie, Z. et al. Structure and lithium storage performances of nickel hydroxides synthesized with different nickel salts. Ionics 23, 1625–1636 (2017). https://doi.org/10.1007/s11581-017-1983-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-1983-3