Abstract
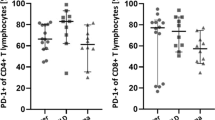
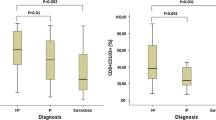
Deepened flow cytometry (FCM) analysis of bronchoalveolar lavage fluid (BALF) cells can disclose clonal B and abnormal T lymphocytes in case of lung involvement in non Hodgkin lymphoma (NHL). The possible role of routine FCM BALF analysis in diagnosing NHL involving the lungs is largely undetermined. To evaluate whether differences exist, within FCM screening of lymphocyte subsets, between BALFs from lung NHL and BALFs from reactive diseases. We compared alveolar leukocyte and lymphocyte data obtained using flow cytometry in 17 lung NHL cases with the median corresponding data detected in 208 controls, matched with cases for computed tomography findings. Absolute leukocyte counts did not differ significantly between cases and controls. As calculated within leukocytes, percentages of total, B, T, CD4+ and CD8+ T lymphocytes, respectively, were significantly higher in B cell NHL cases than in their controls (P = .003, .023, .009, .004, and .020, respectively). Similarly, percentages of total, and CD8+ T lymphocytes, respectively, were significantly higher in T cell NHL cases than in their controls (P = .046 and .027, respectively). Huger BALF lymphocytosis occurs in pulmonary NHLs than in other lung diseases. Possible lymphocyte cutoffs, that could indicate lung NHL and the subsequent need of a second-step BALF staining shortly following the initial screening, should be prospectively attempted.
Similar content being viewed by others
References
Bae YA, Lee KS (2010) Cross-sectional evaluation of thoracic lymphoma. Thorac Surg Clin 20:175–186
Baser S, Onn A, Lin E, Morice RC, Duvic M (2007) Pulmonary manifestations in patients with cutaneous T cell lymphomas. Cancer 109:1550–1555
Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M (2005) Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22:175–184
Carreras J, Lopez-Guillermo A, Roncador G (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27:1470–1476
Cesana C, Klersy C, Scarpati B, Brando B, Volpato E, Bertani G, Faleri M, Nosari A, Cantoni S, Ferri U, Scampini L, Barba C, Lando G, Morra E, Cairoli R (2010) Flow cytometry vs cytomorphology for the detection of hematologic malignancy in body cavity fluids. Leuk Res 34:1027–1034
Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C (2014) T helper 17 cells play a critical pathogenic role in lung cancer. PNAS 111:5664–5669
Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Yin ZN, Mao XH, Guo G, Shi Y, Zou QM (2012) Role of gamma-delta T cells in host response against Staphylococcus Aureus-induced pneumonia. BMC Immunol 13:38
Cherian SV, Thampy E, Mauzo SH (2014) Primary pulmonary lymphoma: a case of ‘unresolving pneumonia’. Am J Med 127:e3–e4
Dahmoush L, Hijazi Y, Barnes E, Stetler-Stevenson M, Abati A (2002) Adult T-cell leukemia/lymphoma. Cancer 96:110–116
Danila E, Norkūniene J, Jurgauskiene L, Malickaite R (2009) Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clin Respir J 3:214–221
Do KH, Lee JS, Seo JB, Song JW, Chung MJ, Heo JN, Song KS, Lim TH (2005) Pulmonary parenchymal involvement of low-grade lymphoproliferative disorders. J Comput Assist Tomogr 29:825–830
Domagała-Kulawik J, Skirecki T, Maskey-Warzechowska M, Grubek-Jaworska H, Chazan R (2012) Bronchoalveolar lavage total cell count in interstitial lung diseases-does it matter? Inflammation 35:803–809
Ebert S, Becker M, Lemmermann NA, Büttner JK, Michel A, Taube C, Podlech J, Böhm V, Freitag K, Thomas D, Holtappels R, Reddehase MJ, Stassen M (2014) Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog 10(4):e1004100
Fireman E, Vardinon N, Burke M, Spizer S, Levin S, Endler A, Stav D, Topilsky M, Mann A, Schwarz Y, Kivity S, Greif J (1998) Predictive value of response to treatment of T-lymphocyte subpopulations in idiopathic pulmonary fibrosis. Eur Respir J 11:706–711
Fu H, Wang A, Mauro C, Marelli-Berg F (2013) T lymphocyte trafficking: molecules and mechanisms. Frontiers in bioscience (Landmark edition) 18:422–440
Hilchey SP, Rosenberg AF, Hyrien O, Secor-Socha S, Cochran MR, Brady MT, Wang JC, Sanz I, Burack WR, Quataert SA, Bernstein SH (2011) Follicular lymphoma tumor-infiltrating T-helper (T(H)) cells have the same polyfunctional potential as normal nodal T(H) cells despite skewed differentiation. Blood 118:3591–3602
Keicho N, Oka T, Takeuchi K, Yamane A, Yazaki Y, Yotsumoto H (1994) Detection of lymphomatous involvement of the lung by broncholaveolar lavage. Application of immunophenotypic and gene rearrangement analysis. Chest 105:458–462
Khojeini EV, Song JY (2014) Intravascular large B-cell lymphoma presenting as interstitial lung disease. Intravascular large B-cell lymphoma presenting as interstitial lung disease. Case Rep Pathol 2014:928065
Kowal-Bielecka O, Kowal K, Highland KB, Silver RM (2010) Bronchoalveolar lavage fluid in scleroderma interstitial lung disease: technical aspects and clinical correlations: review of the literature. Semin Arthritis Rheum 40:73–88
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149
Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C, Wood B (2012) An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 185:1004–1014
Nehashi Y, Nakano M, Utsumi K, Ichinose Y, Toyama K (1993) Primary pulmonary B-cell lymphoma diagnosed by κ − λ imaging of broncho-alveolar lavage fluid lymphocytes. Intern Med 32:480–483
Papiris SA, Kollintza A, Kitsanta P, Kapotsis G, Karatza M, Milic-Emili J, Roussos C, Daniil Z (2005) Relationship of BAL and lung tissue CD4+ and CD8+ T lymphocytes, and their ratio in idiopathic pulmonary fibrosis. Chest 128:2971–2977
Poletti V, Romagna M, Gasponi A, Baruzzi G, Allen KA (1995) Bronchoalveolar lavage in the diagnosis of low-grade, MALT type, B-cell lymphoma in the lung. Monaldi Arch Chest Dis 50:191–194
Reynolds HY (2009) Present status of bronchoalveolar lavage in interstitial lung disease. Curr Opin Pulm Med 15:479–485
Smith PA, Kohli LM, Wood KL, Hage CA, Twigg HL, Knox KS (2006) Cytometric analysis of BAL T cells labeled with a standardized antibody cocktail correlates with immunohistochemical staining. Cytometry B Clin Cytom 70B:170–178
Song JY, Filie AC, Venzon D, Stetler-Stevenson M, Yuan CM (2012) Flow cytometry increases the sensitivity of detection of leukemia and lymphoma cells in bronchoalveolar lavage specimens. Cytometry B Clin Cytom 82B:305–312
Souza CA, Quan K, Seely J, Kravcik S, Burns B (2009) Pulmonary intravascular lymphoma. J Thorac Imaging 24:231–233
Tøndell A, Rø AD, Åsberg A, Børset M, Moen T, Sue-Chu M (2014) Activated CD8+ T cells and NKT cells in BAL fluid improve diagnostic accuracy in sarcoidosis. Lung 192:133–140
Tutor-Ureta P, Citores MJ, Castejón R, Mellor-Pita S, Yebra-Bango M, Romero Y, Vargas JA (2006) Prognostic value of neutrophils and NK cells in bronchoalveolar lavage of sarcoidosis. Cytometry B Clin Cytom 70:416–422
Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y, He R (2012) BLT1-dependent alveolar recruitment of CD4(+)CD25(+) Foxp3(+) regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med 186:989–998
Winterbauer RH, Lammert J, Selland M, Wu R, Corley D, Springmeyer SC (1993) Bronchoalveolar lavage cell populations in the diagnosis of sarcoidosis. Chest 104:352–361
Zinzani PL, Tani M, Gabriele A, Poletti V, Stefoni V, Alinari L, Musuraca G, Bonifazi F, Pileri S, Tura S, Baccarani M (2003) Extranodal marginal zone B-cell lymphoma of MALT-type of the lung: single-center experience with 12 patients. Leuk Lymphoma 44:821–824
Zompi S, Couderc LJ, Cadranel J, Antoine M, Epardeau B, Fleury-Feith J, Popa N, Santoli F, Farcet JP, Delfau-Larue MH (2004) Clonality analysis of alveolar B lymphocytes contributes to the diagnostic strategy in clinical suspicion of pulmonary lymphoma. Blood 103:3208–3215
Acknowledgments
Authors have no financial interests to disclose. The authors wish to thank Ursula Ferri, Claudia Barba, and Carla Mandrisi, for their relevant technical contribution in immunophenotyping, and doctor Mariangela Mura for retrieving histological data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was not funded.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional research committee and/or with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The institutional committee on human experimentation of the ASST Grande Ospedale Metropolitano Niguarda approved the development of this retrospective study without the request of informed consent.
Rights and permissions
About this article
Cite this article
Cesana, C., Scarpati, B., Brando, B. et al. Flow cytometry analysis of lymphocyte subsets in bronchoalveolar lavage: comparison between lung non-Hodgkin lymphomas and reactive diseases. Comp Clin Pathol 26, 447–454 (2017). https://doi.org/10.1007/s00580-016-2397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2397-8