Abstract
Purpose
Clinical trials commonly use physician-adjudicated adverse event (AE) assessment via the common terminology criteria for adverse events (CTCAE) for decision-making. Patient-reported health-related quality of life (HRQoL) data are becoming more frequent in oncology; however, the relationship between physician-adjudicated AE assessment and HRQoL is understudied.
Methods
Data from a phase II trial (clinicaltrials.gov identifier: NCT01143402) where patients with metastatic uveal melanoma were randomized to receive selumetinib, an oral MEK inhibitor, or chemotherapy were analyzed. Patients reported HRQoL at baseline, after 1 month, and end of treatment (n = 118), whereas physicians adjudicated AEs via CTCAE. Mean HRQoL scores were compared between patient randomization arms, as well as between those patients who did/did not receive dose modifications.
Results
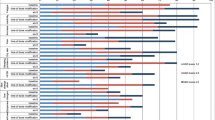
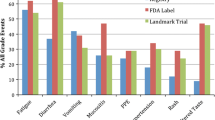
Ninety-four percent had a CTCAE grade ≥1 for at least one treatment-associated AE, with 18% undergoing dose modification due to toxicity. Mean HRQoL scores did not significantly differ at each of the three time points. Patient and physician-adjudicated reports of nausea were significantly correlated at the start (r = 0.31, p < 0.01) and end of treatment (r = 0.42, p < 0.05). There were no significant correlations between need for dose modification and HRQoL scores.
Conclusions
Despite the high rate of physician-adjudicated AEs and need for dose modifications with selumetinib, patient-reported HRQoL was not impacted by treatment. Since HRQoL did not differ in the subgroup of patients who received dosage reductions due to AEs, patients may be willing to tolerate select AEs without dose modification (if medically appropriate). More research is needed to determine how to best integrate HRQoL data into clinical trial conduct.
Similar content being viewed by others
References
Abernethy AP, Zafar SY, Uronis H, Wheeler JL, Coan A, Rowe K, Herndon JE (2010) Validation of the patient care monitor (version 2.0): a review of symptom assessment instrument for cancer patients. J Pain Symptom Manag 40(4):545–558
Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Basch E (2016) The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 24(8):3669–3676. doi:10.1007/s00520-016-3297-9
Basch E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362(10):865–869. doi:10.1056/NEJMp0911494
Basch E (2014) New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med 65:307–317. doi:10.1146/annurev-med-010713-141500
Basch E, Iasonos A, Barz A, Culkin A, Kris MG, Artz D, Schrag D (2007) Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol 25(34):5374–5380. doi:10.1200/JCO.2007.11.2243
Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, Tunis S (2012) Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 30(34):4249–4255. doi:10.1200/JCO.2012.42.5967
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Schrag D (2014) Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. doi:10.1093/jnci/dju244
Basch E, Geoghegan C, Coons SJ, Gnanasakthy A, Slagle AF, Papadopoulos EJ, Kluetz PG (2015) Patient-reported outcomes in cancer drug development and us regulatory review: perspectives from industry, the food and drug administration, and the patient. JAMA Oncol 1(3):375–379. doi:10.1001/jamaoncol.2015.0530
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Schrag D (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34(6):557–565. doi:10.1200/JCO.2015.63.0830
Belknap SM, Georgopoulos CH, West DP, Yarnold PR, Kelly WN (2010) Quality of methods for assessing and reporting serious adverse events in clinical trials of cancer drugs. Clin Pharmacol Ther 88:231–236
Bruner DW, Bryan CJ, Aaronson N, Blackmore CC, Brundage M, Cella D, Whalen G (2007) Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol 25:5051–5057
Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, Schwartz GK (2014) Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 311(23):2397–2405. doi:10.1001/jama.2014.6096
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11(3):570–579
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Group PC (2010) The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63(11):1179–1194. doi:10.1016/j.jclinepi.2010.04.011
Cormier JN, Ross MI, Gershenwald JE, Lee JE, Mansfield PF, Camacho LH, Palmer JL (2008) Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer 112(10):2249–2257. doi:10.1002/cncr.23424
Cornish D, Holterhues C, van de Poll-Franse LV, Coebergh JW, Nijsten T (2009) A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 20(Suppl 6):vi51–vi58. doi:10.1093/annonc/mdp255
Di Maio M, Basch E, Bryce J, Perrone F (2016) Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. doi:10.1038/nrclinonc.2015.222
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, Basch E (2015) Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. doi:10.1001/jamaoncol.2015.2639
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Grob JJ, Amonkar MM, Karaszewska B, Schachter J, Dummer R, Mackiewicz A, Robert C (2015) Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol 16(13):1389–1398. doi:10.1016/s1470-2045(15)00087-x
Hay JL, Atkinson TM, Reeve BB, Mitchell SA, Mendoza TR, Willis G, Group NP-CS (2014) Cognitive interviewing of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res 23(1):257–269. doi:10.1007/s11136-013-0470-1
Jensen RE, Snyder CF (2016) PRO-cision medicine: personalizing patient care using patient-reported outcomes. J Clin Oncol 34(6):527–529. doi:10.1200/jco.2015.64.9491
NCI (2001) National Cancer Institute. Cancer Therapy Evaluation Program. NCI Guidelines—Expedited adverse event reporting requirements for NCI investigational agents. National Cancer Institute, Bethesda
NCI (2010) National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published May 28, 2009; Revised Version 4.03 June 14, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 18 Nov 2016
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse events of cancer treatment. Semin Radiat Oncol 13:176–181
Trotti A, Colevas AD, Setser A, Basch E (2007) Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 25:5121–5127
US Department of Health and Human Services (2014) Guidance for industry and FDA Staff. Qualification process for drug development tools. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm230597.pdf. Accessed 18 Nov 2016
Funding
This project was sponsored by the Cancer Therapy Evaluation Program. Financial support was provided by the National Cancer Institute, the Conquer Cancer Foundation, Cycle for Survival, and the Fund for Opthalmic Knowledge, as well as a National Institutes of Health Support Grant (P30CA08748), which provides partial support for the Behavioral Research Methods Core Facility used in conducting this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The Department of Cancer Treatment and Diagnosis at the National Cancer Institute provided selumetinib for the clinical trial (NCT01143402).
Rights and permissions
About this article
Cite this article
Atkinson, T.M., Hay, J.L., Shoushtari, A. et al. Relationship between physician-adjudicated adverse events and patient-reported health-related quality of life in a phase II clinical trial (NCT01143402) of patients with metastatic uveal melanoma. J Cancer Res Clin Oncol 143, 439–445 (2017). https://doi.org/10.1007/s00432-016-2318-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2318-x