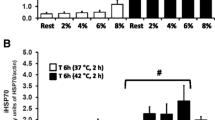
Abstract
Moderate exercise positively impacts innate immune functions, bringing about a better resistance against infections and general immunosurveillance. Exercise of high workloads (i.e., high intensity and/or duration) such as elite marathon, on the other hand, may have detrimental effects over immune function, but neither how long nor how intense should be the exercise sessions to be deleterious is known, this being a matter of intense dispute. Exercise is, at the same time, one of the most powerful inducers of the 70 kDa family of heat shock proteins (HSPAs, formerly known as HSP70s), which are protein chaperones characterized by a marked anti-inflammatory potency, when located intracellularly (iHSPA), but may act as pro-inflammatory cytokines if in the extracellular space (eHSPA). The above observations led us to suppose that short-term exercise could impose long-lasting effects on macrophage function that should be related to the eHSPA-to-iHSPA ratio, viz. H-index. Sedentary adult male Wistar rats were then submitted to 20 min swimming sessions with an overload (as a percentage of body weight attached to the tail base) of either 2, 4, 6, or 8 %. Control animals were maintained at rest in shallow water. Monocyte/macrophage functions (phagocytic capacity, nitric oxide [NO], and hydrogen peroxide [H2O2]) were assessed just after and 12 h after exercise and compared with HSPA status and oxidative stress markers. The results showed that exercise increased phagocytosis and H2O2 immediately after the bouts in a workload-dependent way. This was accompanied by increased H-index but no alteration in the redox status. Enhanced phagocytic capacity persisted for up to 12 h, when a marked rise in NO production was also observed, but H-index resumes its control values, suggesting that immune alertness returned to basal levels. Of note was the detection of the cognate form of eHSPA (encoded by hspa8 gene and formerly known as HSP73) in the rat sera. In total, acute exercise may evoke 12 h long workload-dependent effects associated with HSPA status.






Similar content being viewed by others
References
Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P (2011) Position statement. Part one: immune function and exercise. Exerc Immunol Rev 17:6–63
Silveira EMS, Rodrigues MF, Krause MS, Vianna DR, Almeida BS, Rossato JS, Oliveira LP Jr, Curi R, Homem de Bittencourt PI Jr (2007) Acute exercise stimulates macrophage function: possible role of NF–κB pathways. Cell Biochem Funct 25:63–73
Rossato JS, Krause M, Fernandes AJ, Fernandes JR, Seibt IL, Rech A, Homem de Bittencourt PI Jr (2014) Role of alpha- and beta-adrenoreceptors in rat monocyte/macrophage function at rest and acute exercise. J Physiol Biochem 70:363–374. doi:10.1007/s13105-013-0310-3
Dimauro I, Mercatelli N, Caporossi D (2016) Exercise-induced ROS in heat shock proteins response. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2016.03.028
Chen HW, Kuo HT, Wang SJ, Lu TS, Yang RC (2005) In vivo heat shock protein assembles with septic liver NF-κB/I-κB complex regulating NF-κB activity. Shock 24:232–238
Gutierrez LL, Maslinkiewicz A, Curi R, Homem de Bittencourt PI Jr (2008) Atherosclerosis: a redox-sensitive lipid imbalance suppressible by cyclopentenone prostaglandins. Biochem Pharmacol 75:2245–2262
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6:386–393
Campisi J, Fleshner M (2003) Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J Appl Physiol 9:43–52
Gelain DP, de Bittencourt Pasquali MA, Comim CM, Grunwald MS, Ritter C, Tomasi CD, Alves SC, Quevedo J, Dal-Pizzol F, Moreira JCF (2011) Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock 35:466–470. doi:10.1097/SHK.0b013e31820fe704
Heck TG, Schöler CM, Homem de Bittencourt PI Jr (2011) HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct 29:215–226. doi:10.1002/cbf.1739
Krause MS, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, Homem de Bittencourt PI Jr (2015) The chaperone balance hypothesis: the importance of the Extracellular to Intracellular HSP70 Ratio (eHSP70/iHSP70) to inflammation-driven type 2 diabetes, the effect of exercise and the implications for clinical management. Mediat Inflamm 2015:249205. doi:10.1155/2015/249205
Kregel KC, Allen DL, Booth FW, Fleshner M, Henricksen E, Musch TI, O’Leary DS, Parks CM, Poole DC, Ra’anan AW, Sheriff DD, Sturek MS, Toth LA (2006) Resource book for the design of animal exercise protocols. American Physiological Society, Bethesda
Brown ET, Umino Y, Loi T, Solessio E, Barlow R (2005) Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci 22:615–618
Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA (2005) Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med 230:777–784
Chang Y, Chen TL, Sheu JR, Chen RM (2005) Suppressive effects of ketamine on macrophage functions. Toxicol Appl Pharmacol 204:27–35
Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK (2004) Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein. Cell Stress Chaperones 9:390–396
Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E (2001) Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A: Mol Integr Physiol 130:21–27
Voltarelli FA, Gobatto CA, de Mello MA (2002) Determination of anaerobic threshold in rats using the lactate minimum test. Braz J Med Biol Res 35:1389–1394. doi:10.1590/S0100-879X2002001100018
Bϕyum A, Lϕvhaug D, Tresland L, Nordlie EM (1991) Separtaion of leucocytes: improved cell purity by fine adjustment of gradient medium density and osmolality. Scand J Immunol 34:697–712
El-Sayed MS (1998) Effects of exercise and training on blood rheology. Sports Med 26:281–292
Brun JF, Khaled S, Raynaud E, Bouix D, Micallef JP, Orsetti A (1998) The triphasic effects of exercise on blood rheology: which relevance to physiology and pathophysiology? Clin Hemorheol Microcirc 19:89–104
Pires de Melo M, Curi TC, Miyasaka CK, Palanch AC, Curi R (1998) Effect of indole acetic acid on oxygen metabolism in cultured rat neutrophil. Gen Pharmacol 31:573–578
Grunwald MS, Pires AS, Zanotto-Filho A, Gasparotto J, Gelain DP, Demartini DR, Schöler CM, Homem de Bittencourt PI Jr, Moreira JCF (2014) The oxidation of HSP70 is associated with functional impairment and lack of stimulatory capacity. Cell Stress Chaperones 19:913–925. doi:10.1007/s12192-014-0516-5
Hishikawa T, Cheung JY, Yelamarty RV, Knutson DW (1991) Calcium transients during Fc receptor-mediated and nonspecific phagocytosis by murine peritoneal macrophages. J Cell Biol 115:59–66
Akerboon TPM, Sies H (1981) Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol 77:373–382
Schaun MI, Dipp T, Rossato JS, Wilhelm EN, Pinto R, Rech A, Plentz RD, Homem de Bittencourt PI Jr, Reischak-Oliveira A (2011) The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct 29:534–542. doi:10.1002/cbf.1781
Miragem AA, Ludwig MS, Heck TG, Baldissera FG, dos Santos AB, Frizzo MN, Homem de Bittencourt PI Jr (2015) Estrogen deprivation does not affect vascular heat shock response in female rats: a comparison with oxidative stress markers. Mol Cell Biochem 407:239–249. doi:10.1007/s11010-015-2472-5
Arab K, Steghens JP (2004) Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem 325:158–163
Jiang ZY, Woollard AC, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26:853–856
Södergren E, Nourooz-Zadeh J, Berglund L, Vessby B (1998) Re-evaluation of the ferrous oxidation in xylenol orange assay for the measurement of plasma lipid hydroperoxides. J Biochem Biophys Methods 37:137–146
Lapenna D, Cuccurullo F (1993) TBA test and “free” MDA assay in evaluation of lipid peroxidation and oxidative stress in tissue systems. Am J Physiol 265(3 Pt 2):H1030–H1032
Ceconi C, Cargnoni A, Pasini E, Condorelli E, Curello S, Ferrari R (1991) Evaluation of phospholipid peroxidation as malondialdehyde during myocardial ischemia and reperfusion injury. Am J Physiol 260(4 Pt 2):H1057–H1061
Kolberg A, Rosa TG, Puhl MT, Scola G, da Rocha Janner D, Maslinkiewicz A, Lagranha DJ, Heck TG, Curi R, Homem de Bittencourt PI Jr (2006) Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct 24:23–39
Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, Homem de Bittencourt PI Jr (2015) Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring) 23:120–129. doi:10.1002/oby.20919
Fortes MA, Marzuca-Nassr GN, Vitzel KF, da Justa Pinheiro CH, Newsholme P, Curi R (2016) Housekeeping proteins: how useful are they in skeletal muscle diabetes studies and muscle hypertrophy models? Anal Biochem 504:38–40. doi:10.1016/j.ab.2016.03.023
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Krause M, Bock PM, Takahashi HK, Homem de Bittencourt PI Jr, Newsholme P (2015) The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci (Lond) 128:789–803. doi:10.1042/CS20140695
Pedersen BK, Rohde T, Ostrowski K (1998) Recovery of the immune system after exercise. Acta Physiol Scand 162:325–332
Eijsvogels TM, Thompson PD (2015) Exercise is medicine: at any dose? JAMA 314:1915–1916. doi:10.1001/jama.2015.10858
Moynagh PN (2005) The NF-kappaB pathway. J Cell Sci 118(Pt 20):4589–4592
Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52:741–744
Gwinn MR, Vallyathan V (2006) Respiratory burst: role in signal transduction in alveolar macrophages. J Toxicol Environ Health B Crit Rev 9:27–39
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212
Raftos JE, Whillier S, Kuchel PW (2010) Glutathione synthesis and turnover in the human erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem 285:23557–23567
Gladwin MT, Lancaster JR, Freeman BA, Schechter AN (2003) Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nat Med 9:496–500
Jia L, Bonaventura C, Bonaventura J, Stamler JS (1996) S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380:221–226
Krause MS, Oliveira Junior LP, Silveira EMS, Vianna DR, Rossato JS, Almeida BS, Rodrigues MF, Fernandes AJ, Costa JA, Curi R, Homem de Bittencourt PI Jr (2007) MRP1/GS-X pump ATPase expression: is this the explanation for the cytoprotection of the heart against oxidative stress-induced redox imbalance in comparison to skeletal muscle cells? Cell Biochem Funct 25:23–32
Milne KJ, Noble EG (2002) Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol 93:561–568
Whitham M, Laing SJ, Jackson A, Maassen N, Walsh NP (2007) Effect of exercise with and without a thermal clamp on the plasma heat shock protein 72 response. J Appl Physiol 103:1251–1256
Demirel HA, Powers SK, Naito H, Tumer N (1999) The effects of exercise duration on adrenal HSP72/73 induction in rats. Acta Physiol Scand 167:227–231
González B, Manso R (2004) Induction, modification and accumulation of HSP70 s in the rat liver after acute exercise: early and late responses. J Physiol 556(Pt 2):369–385
McGivney BA, Eivers SS, MacHugh DE, MacLeod JN, O’Gorman GM, Park SD, Katz LM, Hill EW (2009) Transcriptional adaptations following exercise in thoroughbred horse skeletal muscle highlights molecular mechanisms that lead to muscle hypertrophy. BMC Genomics 10:638. doi:10.1186/1471-2164-10-638
Wang R, Town T, Gokarn V, Flavell RA, Chandawarkar RY (2006) HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J Surg Res 136:58–69
Homem de Bittencourt PI Jr, Curi R (2001) Antiproliferative prostaglandins and the MRP/GS-X pump role in cancer immunosuppression and insight into new strategies in cancer gene therapy. Biochem Pharmacol 62:811–819
Acknowledgements and funding
PIHBJ is responsible for grant support with respect to Brazilian National Council for Scientific and Technological Development (CNPq, Grants #402626/2012–5 and #402364/2012–0). TGH was supported by CNPq [#382692/2011–0] and State of Rio Grande do Sul Foundation for Research Support (FAPERGS, grant # 002106–2551/13–5). CMS was recipient of a scholarship from CAPES (The Brazilian Ministry of Education Coordination for the Improvement of Higher Education Personnel).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
CMS, TGH, LPOJ, and PIHBJ designed the study. CMS, CVM, GSS, and TGH completed all the experiments described in this manuscript. CMS, LPOJ, TGH, and PIHBJ analyzed the results. CMS and PIHBJ co-wrote the article. PIHBJ provided experimental advice and revised the final version of the manuscript. All the authors had final approval of the submitted and published versions.
Conflicts of interest
The authors declare no conflict of interest and no competing interests such as consultancies, financial involvement, patent ownership, etc. in relation to the work described herein. CNPq, FAPERGS and CAPES (the funding organisms) had no involvement in the propositions presented in this manuscript.
Ethical approval
All the procedures performed in studies involving the animals followed the ethical rules established by Arouca’s Act (Federal Law 11794/2008) and the Guide for Care and Use of Experimental Animals published by the National Institutes of Health (NIH publication no. 85-23, revised in 1996). The procedures were approved by the Federal University of Rio Grande do Sul Ethics Committee on Animal Experimentation (CEUA #23451), according to the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA).
Rights and permissions
About this article
Cite this article
Schöler, C.M., Marques, C.V., da Silva, G.S. et al. Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol Cell Biochem 421, 111–125 (2016). https://doi.org/10.1007/s11010-016-2791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2791-1