Abstract
N,N-Diethyl-hydroxylamine (DEHA) is a novel salt-free reductant used in the reprocessing of spent fuel, this paper reports on γ-rays damage of 0.1–0.5 mol L−1 DEHA irradiated 5–25 kGy and its radiolytic products. DEHA is relative stable against radiation, its radiolysis rate increases with the dose, but decreases with increasing initial DEHA concentration. The main radiolytic products are hydrogen, ethene, methane, acetaldehyde and ammonium ion, their quantities increase with initial DEHA concentration. Hydrogen and liquid radiolytic products quantities increase with the dose, the dependence of hydrocarbon quantity on dose relates to original DEHA concentration and dose.
Similar content being viewed by others
Introduction
With the development of society, the demand for energy continues to increase world widely. As a kind of clean energy, nuclear energy has been used for tens of years. However, during the use of nuclear energy, a lot of spent nuclear fuel is produced. The spent fuel contains most of the fertile material (U238) that was present in the fuel when charged, appreciable concentration of valuable fissile nuclides (U235 and Pu239) and large amounts of radioactive fission products. If spent fuel enters our biosphere, it will severely contaminate our environment and harm people’s health. On the other side, if we can reprocess the spent fuel efficiently, we can recover U and Pu for reuse as nuclear fuel, remove long life radioactive nuclides and convert them into forms suitable for safe and long-term storage. The Purex process has been the most matured method for spent fuel recovery, in this process, U (VΙ) and Pu(IV) are coextracted from an aqueous feed solution of 2–3 mol L−1 nitric acid into a solution of 30 % tributylphosphate (TBP) in kerosene, and then Pu(IV) is reduced to organic-insoluble Pu(III) by a selective reducing reagent, while U (VI) in organic phase is not affected, thus Pu is separated from U [1, 2]. Ferrous sulfamate [Fe(NH2SO3)2] was used as a reductant in separation of Pu from U in early plants [3–6], it can rapidly reduce Pu(IV) to Pu(III), however, the use of this reductant adds a large amount of nonvolatile ferric sulfate into the radioactive effluents which obviously increases the cost of final disposal of radioactive waste, and sulfate ion causes stainless steel components corrode. In order to overcome the drawbacks of Fe(NH2SO3)2, hydrazine-stabilized U(IV) was developed [3, 7]. U (IV)–N2H4 reduces Pu(IV) to Pu(III) very fast, U (IV) is converted to U (VI), which is extracted by the organic stream, the application of U (IV)–N2H4 introduces no nonvolatile or corrosive constituents into the process, but its application has the risk of potential production of explosive HN3 caused by the reaction of N2H4 with nitrous acid. Mckibben [8] reports that hydroxylamine nitrate (NH2OH–HNO3) can quickly and completely reduce Pu(IV) to Pu(III), and in process waste streams, NH2OH–HNO3 can entirely decompose into water and gases at higher temperature, leaving no salt, so it is salt-free reductant. However, the reaction rate between Pu(IV) and NH2OH sharply decreases with increasing acidities, therefore it can’t be used in the separation of Pu from U in the first solvent extraction. On the other hand, there is Neptunium (Np) in power reactor spent fuel, none of above reductants can control the valence of Np, and this makes Np go to different phases [9]. In order to overcome the shortcomings of above reductants, many studies have been made. N,N-diethyl-hydroxylamine [DEHA, (C2H5)2NOH)] is a derivative of NH2OH, and it is also a kind of salt-free reductant, it is of appropriate reducing ability and excellent selectivity. Reports [10–12] show that DEHA not only reduces Np(VI) and Pu(IV) to Np(V) and Pu(III) that can not be extracted by TBP, but also stabilizes Np(V)and Pu(III) for a period of time in certain conditions [13]. The application of DEHA brings about none of nonvolatile, corrosive constituents and HN3 into the process, so DEHA is a promising reductant. Since the separation of Pu and Np from U is processed in radiation field, while organics are commonly sensitive to radiation, radiation damage of DEHA and its radiolytic products must be studied before its application. Reports [14–17] show that at higher dose to 1000 kGy, DEHA radiolytic rate is very high, and the relationships of radiolytic gas and organics quantity on dose and initial DEHA concentration are complicated. Another report [18] calculates that reagents used in the reprocess of spent fuel usually absorb about 10 kGy radiation energy, in order to give reliable reference to the application of DEHA in the reprocessing of spent fuel, we studied the radiolysis of DEHA at 5–25 kGy. In this paper, in addition to radiation damage of DEHA and its radiolytic gas and liquid organics, we also studied ammonium ion generated by radiolysis of DEHA aqueous solution.
Experimental
DEHA and standard gas mixture
DEHA is supplied by China Institute of Atomic Energy and its purity is 98.6 %. Standard gas mixture is supplied by Shanghai Institute of Measurement and Testing Technology, and is composed of 0.5 % hydrogen, 0.03 % methane, 0.03 % ethane, 0.03 % ethene, 0.03 % propane, 0.03 % propene, 0.03 % n-butane and 99.32 % nitrogen.
Main equipment and accessories
60Co γ-rays radiation facility: 1.295 × 1015 Bq, Shanghai Institute of Applied Physics, Chinese Academy of Sciences; GC7890A gas chromatograph with 7683 automatic liquid sampler and 30 m × 0.32 mm DB-FFAP capillary column: supplied by Agilent Technologies Co. Ltd.; GC900A gas chromatograph and 2 m × 3 mm packed 5 Å molsieve column: Shanghai Ke Chang Chromatograph Instruments Co. Ltd.; 50 m × 0.53 mm fused-silica PLOT Al2O3 column, supplied by Lanzhou Institute of Chemistry and Physics, Chinese Academy of Science; Metrohm ion chromatograph (research-style): supplied by Switzerland Metrohm Co. Ltd..
Sample preparation and irradiation
0.1, 0.2 and 0.5 mol L−1 DEHA solutions were prepared with high purity water, 4 ml solutions were taken and placed into 7 ml penicillin bottles, and then the bottles were sealed with a sealing machine. These samples were irradiated by 60Co γ-rays to 5, 10, 15, 20 and 25 kGy. The doses were monitored by dichromate dosimeters.
Analysis methods of DEHA and its radiolytic products
The analysis of DEHA, acetaldehyde, ethanol, acetic acid and nitroethane was performed by gas chromatography in which a FFAP capillary column and a flame ionization detector (FID) were equipped [14], carrier gas is N2, and its flow is 25 mL min−1; column temperature is programmed: initial temperature: 60 °C, initial isothermal period: 5 min, programmed heating rate: 6 °C min−1, final temperature: 100 °C, final isothermal period: 5 min. FID temperature: 150 °C. The analysis of H2 was performed by gas chromatography in which 5 Å molsieve packed column and thermal conductivity detector (TCD) were equipped [17], carrier gas is Ar, and its flow is 10 mL min−1; column temperature is 85 °C, TCD temperature is 110 °C. The analysis of light hydrocarbon was also made by gas chromatography, but the column was PLOT Al2O3 column, and the detector was FID [15], carrier gas is N2, and its flow is 30 mL min−1; column temperature is 65 °C, FID temperature is 110 °C.
The analysis of acetic acid is also conducted by ion chromatography, the column is METROSEP A SUPP 5–250, the elute is a solution containing 3.2 mmol L−1 Na2CO3 and 1.0 mmol L−1 NaHCO3, and its flow is 0.7 ml min−1. The analysis of ammonium ion is carried out by ion chromatography, the column is METROSEP C2-250, the elute is 2.0 mmol L−1 HNO3, and its flow is 1.0 ml min−1.
Formulae used in the paper
The radiolysis rate of DEHA is defined as follows:
where C o is DEHA concentration in controlled DEHA sample (mol L−1), C i is DEHA concentration in irradiated DEHA sample (mol L−1).
The volume fraction of gas products is calculated as follows: If the response curve equation of a component is y = ax + b, X axis is standard gas mixture volume injected (μL), Y axis is the corresponding peak area of the component (μv*s), then the volume fraction of the component is calculated by follow formula:
where A is the component peak area in the gas chromatogram of the gas sample evolved from irradiated DEHA solution (μv*s), c is volume fraction of the component in the standard gas mixture, e is sample gas volume injected (μL).
Results and discussion
Radiation damage of DEHA in water
The analysis of DEHA was performed by gas chromatography in which a FFAP capillary column and a FID were equipped [14], the response curve for DEHA is y = 17684.97x − 100.44 (concentration range: 0.01–0.5 mol L−1), and the correlation coefficient (R 2) is 1.00. The radiolysis rate of DEHA as a function of dose is shown in Fig. 1.
When the absorbed dose of DEHA solution is 5–25 kGy, the radiolysis rate of 0.2 and 0.5 mol L−1 DEHA is 0.060(13) %–5.2(11) %, and that of 0.1 mol L−1 DEHA is 4.6(10)–16.6(3) %. The radiolysis rate increases with the dose; for 0.1 mol L−1 DEHA, the radiolysis rate increases slowly with the dose at lower dose, but increases sharply at higher dose, however, this increasing is not obvious when the dose is higher than 20 kGy. The radiolysis rate decreases with increasing initial DEHA concentration. The dependence of DEHA radiolysis rate on dose and initial DEHA concentration at 5–25 kGy is similar to that at higher dose to 1000 kGy [16], but the radiolytic rate in the latter case is much higher.
Radiolytic products of DEHA solution
According to the theory of radiation chemistry and organic chemistry, the radiolytic products of DEHA solution may include hydrogen, light hydrocarbon (methane, ethane, ethene, propane, propene and n-butane) and organics (acetaldehyde, ethanol, acetic acid, nitroethane and N-ethylhydroxylamine) [14, 17]. When dilute aqueous organics solution is irradiated, the observed chemical changes are mainly brought about by the reaction between the active species produced by water radiolysis and solute. When water is irradiated, active species H·, ·OH, e −aq and others are formed [19, 20]:

·H and e −aq are reductive, while ·OH is oxidative, as DEHA is a medium reductant, it should be more likely to react with ·OH. Saunders [21] reports that the rate constant of ·OH reaction with DEHA is much larger than that of e −aq reaction with DEHA. H· is a medium reductant, the reaction rate of DEHA with H· should slower than that with e −aq , report [22] shows that the rate constant of DEHA reaction with ·H is much smaller than that with e −aq , so the most important reaction between DEHA and active species generated by water radiolysis is the reaction of DEHA and ·OH. ·OH can react with DEHA by H abstraction to give N,N-diethyl nitroxide radical [21]:

Diethyl nitroxide radical self-reacts to produce nitrone, which hydrolyzes to give acetaldehyde and N-ethylhydroxylamine [23]:


NH2OH can decompose into ammonium ion:

So the radiolytic products of DEHA solution may include hydrogen, light hydrogen, organics and ammonium ion.
H2 produced by irradiated DEHA solution
The analysis of H2 was performed by gas chromatography in which 5 Å molsieve packed column and TCD were equipped [17], results show that there is H2 in the gas sample generated from irradiated DEHA solution, the response curve of H2 is y = 475254x + 26844 (volume range of standard gas mixture: 0.10–5.0 mL), R 2 is 0.9992. The volume fraction of H2 as a function of dose is shown in Fig. 2.
For (0.1–0.5) mol L−1 DEHA irradiated 5–25 kGy, the volume fraction of H2 is 1.43(14) × 10−3 − 27.5(3) × 10−3. H2 volume fraction increases quickly with the dose, it also increases with initial DEHA concentration. While the same concentration DEHA solutions are irradiated at higher dose to 1000 kGy [17], H2 volume fraction also increases with the dose, but the volume fraction of H2 generated from irradiated DEHA solution at higher dose to 1000 kGy is much higher than that at 5–15 kGy, and the relationship between H2 volume fraction and initial DEHA concentration is not obvious. When DEHA solution is irradiated, water in solution will radiolyze to produce H· radical [Eq. (3)], H· may react with DEHA to produce H2, it may also react with each other to generate H2:


At lower dose (such as 5–25 kGy), H· concentration is not high, H2 may mainly generated by the reaction of H· with DEHA [Eq. (8)], so H2 volume fraction increases with initial DEHA concentration. On the other hand, the higher the dose, the higher the concentration of H·[Eq. (3)], this is why H2 volume fraction increases with the dose. At higher dose to 1000 kGy, H· concentration is very high, H2 may mainly produced by the recombination of H·[Eq. (9)], as H· is mainly generated by water radiolysis, H2 volume fraction increases with the dose, but changes little with increasing DEHA concentration.
Light hydrocarbon generated by irradiated DEHA solution
The analysis of light hydrocarbon was also made by gas chromatography, but the column was Al2O3 PLOT column, and the detector was FID [15], results show that there are methane, ethane, ethene, propane, propene and n-butane in the gas evolved from irradiated DEHA solutions. The response curves (volume range of standard gas mixture: 20.0–400.0 μL) and R 2 for hydrocarbon analysis are listed in Table 1.
The volume fraction of light hydrocarbon produced by irradiated DEHA solution as a function of dose is shown in Fig. 3.
The volume fraction ranges of CH4, C2H6, C2H4, C3H8, C3H6 and n-C4H10 are shown in Table 2.
From Table 2, we can see that the highest volume fraction is C2H4, the second and the third are CH4 and C2H6 separately, the volume fractions of C3H8, C3H6 and n-C4H10 are much lower than those of C2H4, CH4 and C2H6, this agrees with the light hydrocarbon generated by radiolysis of the same concentration DEHA solutions at higher dose to 1000 kGy [17], But the volume fraction of hydrocarbon generated by irradiated DEHA solution at 5–25 kGy is much lower than that at higher dose to 1000 kGy. The volume fraction of CH4, C2H4, C3H8, C3H6 and n-C4H10 increases with initial DEHA concentration, but that of C2H6 decreases with increasing initial DEHA concentration. As to 0.2 and 0.5 mol·L−1 DEHA, the volume fraction of hydrocarbon increases with the doses; however, for 0.1 mol L−1 DEHA, the volume fraction of hydrocarbon increases with the doses at lower dose, but changes little at dose higher than 20 kGy. Light hydrocarbon mainly comes from the direct reaction of γ-rays on DEHA in water [19], When DEHA solution is irradiated, DEHA molecules get excited, and this may lead to the breaking of C–N and C–C bonds, thus ·CH3 and ·CH2CH3· are produced, and they may react with DEHA by H abstraction to generate CH4 and CH3CH3:



Since bond energy of C–N is lower than that of C–C, C–N should be more easily be broken than C–C, and the concentration of ·CH2CH3 should be higher than that of ·CH3. In addition to react with DEHA to form ethane, ·CH2CH3 may react with other radicals to form ethene as follows:



Because of above competing reactions, the volume fraction of ethane is considerably lower than that of methane although the primary concentration of ethyl is higher than that of methyl, and the volume fraction of ethene is much higher than that of ethane. On the other hand, propane, propene and n-butane are mainly generated by the reactions between·CH3 and ·CH2CH3 or two ·CH2CH3 radicals, while methane and ethane are produced by the reactions of DEHA with ·CH3 and ·CH2CH3 (Eqs. 11–12), since the concentration of ·CH3 and ·CH2CH3 is much lower than that of DEHA, the volume fraction of propane, propene and n-butane is much lower than that of methane and ethane.
Organics produced by irradiated DEHA solution
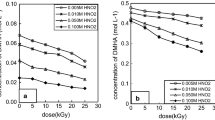
The liquid organics in irradiated DEHA solution may be DEHA, acetaldehyde, ethanol, acetic acid, nitroethane and N-ethylhydroxylamine, they can be analyzed by gas chromatography, in which a FFAP capillary column and a FID detector were equipped [14]. On the other hand, acetic acid can be changed into acetic anion in alkaline solution, so it can also be analyzed by ion chromatography. Our experiment results show that the lowest detectable concentration of acetic acid analyzed by gas chromatography is higher than that analyzed by ion chromatography, since the concentration of acetic acid should be low in irradiated DEHA solution, we applied ion chromatography to quantitative analysis acetic acid in sample solutions. Results show that there are DEHA, acetaldehyde and acetic acid in irradiated DEHA solutions. The response curve for acetaldehyde is y = 3.9343x + 0.5349, concentration range is (0.5–10.0) × 10−3 mol L−1, and R 2 is 0.9996; the response curve for acetic acid is y = 3379.9x + 84.694, concentration rang is (0.017–1.67) × 10−3 mol L−1, and R 2 is 0.9993. The concentration of liquid organics produced by irradiated DEHA solution as a function of dose is shown in Fig. 4.
Figure 4 shows that for 0.1–0.5 mol L−1 DEHA irradiated 5–25 kGy, the concentration of CH3CHO and CH3COOH is 1.24(4) × 10−3 to 7.74(23) × 10−3 and 0.48(10) × 10−3 to 1.65(3) × 10−3 mol L−1 separately, the concentration of CH3CHO is higher than that of CH3COOH, CH3CHO and CH3COOH concentrations increase with the dose, as well as initial DEHA concentration.
As to the same concentration DEHA solutions irradiated at higher dose to 1000 kGy [16], the concentration of CH3CHO is also higher than that of CH3COOH at dose lower than 100 kGy, but CH3CHO and CH3COOH concentrations are higher than those produced by irradiated DEHA solution at 5–25 kGy. The dependence of CH3CHO concentration on initial DEHA concentration at higher dose to 1000 kGy is similar to that at 5–25 kGy. CH3CHO concentration increases with the dose at dose lower than 500 kGy, but decreases with increasing dose at dose higher than 500 kGy. The dependences of CH3COOH concentration on initial DEHA concentration and dose are complicated.
Ammonium ion produced by irradiated DEHA solution
NH4 + is often analyzed by Nessler’s reagent spectrophotometry [24, 25] and ion chromatography [26], our experiment results show that DEHA interferes with the analysis of NH4 + by Nessler’s reagent spectrophotometry, so we choose ion chromatography to analyze NH4 +. The response curve for NH4 + is y = 121.11x + 26.12, concentration range is (0.6–277.8) × 10−5 mol L−1, and R 2 is 0.9993. The concentration of NH4 + as a function of dose is shown in Fig. 5.
Figure 5 shows that for 0.1–0.5 mol L−1 DEHA irradiated 5–25 kGy, NH4 + concentration is 1.05(21) × 10−3 to 5.52(9) × 10−3 mol L−1, NH4 + concentration increases with the dose and initial DEHA concentration.
Conclusions
N,N-Diethyl-hydroxylamine (DEHA), a novel salt-free reducing reagent used in the reprocessing of spent nuclear fuel, is relatively stable in water at dose up to 25 kGy, and its radiation damage can be decreased by increasing its initial concentration. The main radiolytic products are hydrogen, ethene, methane, acetaldehyde and ammonium ion, and their quantities increase with initial DEHA concentration. The quantities of hydrogen, acetaldehyde and ammonium ion increase with the dose; the dependence of hydrocarbon quantity on dose relates to the dose and original DEHA concentration. Since the reprocessing of spent fuel is operated in nitric acid, the effect of nitric acid on radiolytic damage of DEHA and its radiolytic products must be studied, this research has been finished, and the writing of relative paper is under consideration.
References
Benedict M, Pigford TH, Levi HW (1981) Nuclear chemical engineering. McGraw-Hill Book Company, New York, pp 455–473
Choppin GR, Morgenstern A (2000) The PUREX process, using a solution of 30% TBP in kerosene as the organic phase. Radionuclide separations in radioactive waste disposal. J Radioanal Nucl Chem 243(1):45–51
Jiang ShJ, Ren FY (1995) Nuclear fuel reprocessing technology. Atomic Energy Publisher, Beijing, pp 93–104
Biddle P, Miles JH (1968) Rate of reaction of nitrous acid with hydrazine and with sulphamic acid. J Inorg Nucl Chem 30:1291–1297
Perrott JR, Stedman G (1977) The kinetics of nitrite scavenging by hydrazine and hydrazoic acids at high acid acidities. J Inorg Nucl Chem 39(2):325–327
Dukes EK, Wallace RM (1962) Formation of hydrazoic acid from hydrazine in nitric acid solutions. DP-728:1–15
Schlea CS, Caverly MR, Henry HE, Jenkins WJ (1963) Uranium(IV) nitrate as a reducing agent for plutonium(IV) in the PUREX process. DP-808:1-20
Mckibben JM, Bercaw JE (1971) Hydroxylamine nitrate as a Plutonium reductant in the PUREX solvent extraction process. DP-1248:1–22
Ochsenfeld W, Petrich G (1983) Neptunium decontamination in a uranium purification cycle of a spent fuel reprocessing plant. Sep Sci Technol 18(14&15):1685–1698
Koltunov VS (1998) Stabilization of Pu and Np valences in Purex Process: problems and outlook. In: RECOD’98, the 5th international nuclear conference on recycling, condition and disposal, The French Nuclear Society and the European Nuclear Society, Nice, 425–431
Koltunov VS, Baranov SM, Zharova TP (1993) Kinetics of the reactions of Np and Pu ions with hydroxylamine derivatives (VI)—reaction between Np (VI) and N, N–diethylhydroxylamine. Radiokhimiya 35:79–84
Zhang AY, Liu Y (2000) Hydroxylamine derivative in Purex Process III. The kinetics of oxidation-reduction reaction between N,N-diethylhydroxylamine and neptunium(VI). J Radioanal Nucl Chem 245(2):357–361
Sze K, Gosselin JA (1983) Oxidation of Pu (III) by nitric acid in tri-n-butyl phosphate solutions. Part II. Chemical methods for the suppression of oxidation to improve plutonium separation in contactor operation. Nucl Technol 63:431–441
Wang JH, Wan Y, Wu MH, Bao BR, Sun XL, Zheng WF, Zhang ShD (2008) Analyses of organics in irradiated aqueous N,N-diethyl-hydroxylamine solution. Nucl Sci Tech 19:343–346
Wang JH, Bao BR, Wu MH, Sun XL, Zhang XY, Hu JX, Ye GA (2004) The qualitative and quantitative analysis of the light hydrocarbons produced by radiation degradation of N,N-Diethyl-hydroxylamine. J Radioanal Nucl Chem 262:451–453
Wang JH, Zhang J, Wu MH, Xu G, Bao BR, Sun XL, Zheng WF, He H, Zhang ShD (2011) Radiolytic organics in γ-rays irradiated aqueous solution of N,N-diethylhydroxylamine. Nucl Sci Tech 22(2):95–98
Wang JH, Wang ShX, Bao BR, Li Zh, Li Ch, Zheng WF, Zhang ShD (2008) Gaseous product generated by radiation degradation of N,N-diethylhydroxylamine aqueous solution. Nucl Sci Tech 19(2):79–82
Li HB, Su Zh, Cong HF, Song FL, Wang XR, He H, Liu ZhY, Lin CSh (2012) α and γ irradiation stability of 30%TBP-kerosene-HNO3 systems. J Radioanal Nucl Chem 34(5):280–285
Wu JL, Qi ShCh (1993) Radiation Chemistry. Atomic Energy Publisher, Beijing, pp 156–198
Spinks JWT, Woods RJ (1976) An introduction to radiation chemistry, 2nd edn. Wiley, New York, pp 247–295
Barbara SS, Robert AG Jr (1979) Reactions of diethylhydroxylamine with radiolytically produced radicals in aqueous solution. J Phys Chem 83(13):1696–1701
Ying W (2011) γ Radiolysis of diethylhydroxylamine in nitric acid, pulse radiolysis of diethylhydroxylamine and dimethylhyroxylamine. Dissertation for Master Degree, Shanghai University, 79–102
Adamic K, Bowman DF, Gillan T, Ingold KU (1971) Kinetic applications of electron paramagnetic resonance spectroscopy. I. self-reactions of diethyl nitroxide radicals. J Am Chem Soc 93(4):902–908
Lioret SM, Legua CM, Falco PC (2002) Ammonium determination in water samples by using OPA-NAC reagent: a comparative study with Nessler and ammonium selective electrode methods. Int J Environ Anal Chem 82(7):475–489
Marina MSF, Reis BFD, Fo HB, Baccan N (1992) Flow-injection determination of low levels of ammonium ions in natural waters employing preconcentration with a cation-exchange resin. Anal Chim Acta 261(1–2):339–343
Saigne C, Kirchner S, Legrand M (1987) Ion-chromatographic measurements of ammonium, fluoride, acetate, formate and methanesulphonate ions at very low levels in antarctic ice. Anal Chim Acta 203(1):11–21
Acknowledgments
The authors wish to extend warm acknowledgement for the financial assistance of Natural Science Foundation of China (No. 20771074) and Program for Innovative Research Team in University (No. IRT13078).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J.H., Wang, C.C., Wang, S.X. et al. γ-ray damage of N,N-diethyl-hydroxylamine in water and its radiolytic products at lower dose. J Radioanal Nucl Chem 309, 503–510 (2016). https://doi.org/10.1007/s10967-015-4641-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4641-0