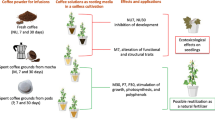
Abstract
Aims
Coffee silverskin (CS) is the most abundant waste from coffee roasting. In this research, the potential of CS as soil fertilizer for barley (Hordeum vulgare L.) cultivation was evaluated.
Methods
Barley plants were grown, for 10 days from the start of germination, in soils mixed with different CS percentages (0, 2, 6 or 10%). The effects of CS addition on soil capacity to retain water and on the mold/yeast community were evaluated. Physio-morphological and biochemical responses of leaves and roots of barley plants growing in CS-enriched soil were evaluated.
Results
Root and shoot length decreased in plants grown with CS, but biomass was less affected and even increased at 2% CS. Photosynthesis was higher in barley seedlings grown on CS-enriched soil than in control plants. However, Chl b was reduced in leaves of plants grown with CS. The total leaf protein content did not change with moderate CS additions in soil. However, when grown in soil with 10% CS, a significant reduction of total protein and Rubisco was observed. Extracts of barley shoots from plants grown in soil with 10% CS showed a greater antioxidant capacity with respect to all other experimental conditions.
Conclusions
We conclude that CS addition to soil may improve soil property to retain water and, at low concentrations (2% w/w) positively affects barley physiology and growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After tea, coffee represents the 2nd most popular beverage in the world, with approximately 500 billion cups being consumed worldwide annually (Ludwig et al. 2014). Global coffee production reached 168.5 million bags in the year 2021/2022; it is expected to increase to 171.3 million bags in 2022/2023 (ICO 2023). Most of the world’s coffee is produced by South America, especially Brazil. However, other countries such as Vietnam, Colombia, Indonesia, Ethiopia, India and Uganda significantly contribute to the world production of coffee (Bessada et al. 2018).
The coffee beverage is obtained after roasting green coffee beans. During this technological phase, the silver-colored thin tegument that covers and protects the outer layer of beans (Coffee Silverskin - CS) is removed and discarded. CS is 4.2% (w/w) of seed total weight representing the unique by-product discarded after coffee roasting (Martuscelli et al. 2021; Toschi et al. 2014). At the moment, CS has no commercial value, and is discarded as a solid and voluminous waste. CS transformation into valuable products, or its utilization in different industrial sectors, however, is becoming very attractive (Overturf et al. 2021). CS composition has been physically characterized and chemically analyzed (Hejna 2021; Lorbeer et al. 2022) also based on the geographical origins of coffee (Bessada et al. 2018). CS contains a high amount of fiber (60–80%), low fat (1.6–3.3%), proteins (19%) and minerals (8% ash). Due to the high content of dietary fiber, the composition of the fiber was investigated in more detail (Hejna 2021; Lorbeer et al. 2022), resulting ~90% insoluble and only 10% soluble (Borrelli et al. 2004; Costa et al. 2018; Jiménez-Zamora et al. 2015). CS is also rich of phenolic compounds mainly chlorogenic acids (CGA) (1–6%), caffeine (0.8–1.25%) and melanoidins (17–23%). Based on its chemical composition and bioactivity, CS has been extensively proposed as a food ingredient (Lorbeer et al. 2022; Nolasco et al. 2022). Coffee phenolics, in particular, have attracted much interest in recent years due to their strong antioxidant and metal-chelating properties (Pourfarzad et al. 2013).
As a case-study plant, barley (Hordeum vulgare L.) was chosen. Barley is a main C3 cereal as distribution area, and an important food source (Araus et al. 2002) The European Union is the world’s leading producer of barley, with an annual production of 52.75 million tons of seeds; in the years 2021/2022 world barley production was 147.05 million tons (Lukinac and Jukić 2022). While in the last century barley was mainly cultivated and used as human food, it is now used for about 60% as animal feed, 30% for malt production in the beer industry, 7% to produce seeds, and only 3% as human food (Baik and Ullrich 2008; Daneri-Castro et al. 2016).
The use of fertilizers containing nitrogen, phosphorus and potassium is considered essential to increase yields of barley and other grasses (Al-Zubade et al. 2021). While the application of chemical fertilizers has improved agricultural yields in response to growing demand, inefficient and inappropriate fertilization practices have led to multi-pronged problems such as degradation of soil quality, groundwater contamination, loss of biodiversity, decrease in soil fertility, development of pest resistances, acidification problems, and other potential agriculture-based pollution episodes (Jiang et al. 2020; Srivastav et al. 2020; Zhang et al. 2022). To cope with these problems, the European Commission has launched the Farm to Fork strategy of the Horizon Europe program, aiming to reduce by 2030 the use of chemical fertilizers (− 20%) and of pesticides (− 50%) (European Commission 2020). To help achieve this objective, current efforts aim to significantly reduce the use of synthetic agrochemicals and to adopt circular production approaches for the valorization of industrial waste. Indeed, the increase in the human population, the reduction of cultivable lands and the climate warming crisis have all arisen the need to develop new agricultural practices with low environmental impact while sustaining crop yield. In this scenario organic fertilizers or biostimulants are promoted to improve plant growth, performances under abiotic stress, fruit set, crop productivity, and nutrient use efficiency, all in an environmentally sustainable way (Francesca et al. 2022; Rouphael and Colla 2020). The high macro and micro-nutrient contents of CS, make it an attractive resource as horticultural fertilizer (Picca et al. 2023).
The aim of this research was to evaluate CS suitability as a fertilizer for barley plants. We specifically evaluated the suitability of CS as a biofertilizer applied to the soil by determining its effects on soil properties, as well as growth and the development of barley plants, leaf protein contents, antioxidant capacity in shoot extracts, and functionality of the photosynthetic apparatus.
Materials and methods
Coffee Silverskin
The CS from Coffea arabica (var. Uganda) was collected from the coffee processing plant of Caffè Cortese S.r.l. located in Acerra (NA). The CS was tested negative for mold and yeast on Rose-Bengal selective agar medium. Inoculated plates were incubated in an upright position at 25 °C ± 1 °C for 5 d. The CS was then ground at 10,200 rpm for 1 min (Bimby mod.TM 5, Vorwerk, Germany) until reaching a particle size of 250–125 μm. The obtained CS powder was mixed at different percentages (see below) to the soil used for barley cultivations.
Treatments and soil properties
For the experiment the commercial soil DORATER by VIGOR PLANT (pH 5.8–6.8; 0.25–0.35 dS m−1 electrical conductivity; 89% soil porosity) was used. Barley seedlings were grown on four different soil conditions: 1) Control (Ctrl): soil not enriched with CS; 2) Soil enriched with 2% CS w/w; 3) Soil enriched with 6% CS w/w; 4) Soil enriched with 10% CS w/w. The addition of CS doesn’t significantly affect the pH of the soil.
To assess possible effect of CS on soil water retention capacity, plastic pots (100 g of soil) were brought to field capacity (FC, g of water/g of soil) by immerging them in water for ∼10 min and were weighed after water drainage ending. The water-saturated pots were then dried for 10 d at room temperature and weighed periodically. The reduction of pot weights was used to calculate the water losses over time. The weight difference between water-saturated and 10 d-dried soil was taken as water field capacity (WFC). The soil water loss in the different conditions was expressed as a percentage, which allowed us to standardize the starting point and to be able to compare the loss of water more adequately over time.
Plant material and growth conditions
Seeds of barley (Hordeum vulgare L. var. Alfeo) were supplied by the Istituto Sperimentale per la Cerealicoltura, Fiorenzuola D’Arda, Italy). Seeds were soaked for 2 h in calcium sulphate (2 mM CaSO4) to initiate germination in absence of pathogens. Seeds germinated after 3–5 d in the dark on moistened distilled water Whatman Paper Circles, 32 mm, in plastic Petri dishes (Carfagna et al. 2011). After germination, the seedlings were placed in the plastic pots, as described earlier. Plants were grown in lab condition (from September 2022 until June 2023) at a temperature of 25 ± 2 °C and a photoperiod of 16/8 hours with a photosynthetic active radiation (PAR) of 50–120 μmol m−2 s−1 during daylight. The seedlings were regularly watered every 2 d with 20 mL of water. All measurements were carried out on 10-d-old seedlings.
Phenotypic (growth) measurements
Fresh weight of entire plants, and of their aboveground and belowground parts was measured using an accurate balance (Mettler Toledo AL54 Analytical Balance, 51 g × 0.1 Mg, 120 V). The roots were accurately cleaned from the soil with water before being weighed. Root and shoot lengths were measured with a digital caliper (Vodlbov Digital Vernier Caliper 0-150 mm).
Chlorophyll contents
The chlorophyll a (Chl-a) and chlorophyll b (Chl-b) contents were estimated spectrophotometrically using N,N-dimethylformamide as a solvent according to (Salbitani et al. 2021). Pigments were extracted in the dark at 4 °C for about 24 h.
Fluorescence parameters measurement
Chlorophyll fluorescence was measured in vivo on whole barley seedlings with an IMAGING-PAM M-Series Chlorophyll Fluorometer (Walz). Plants were kept in the dark for a period of 30 min before analysis. After dark adaptation, the maximal quantum efficiency of PSII in the dark (Fv/fm, where Fv is the variable and Fm is the maximal fluorescence in dark-adapted organisms) was evaluated, and the plants were then exposed to actinic light (AL). AL was increased between 1 and 700 μmol photons m−2 s−1 of PAR at 20 s-long steps, to measure light responses. The parameters measured at each step of the light response were: 1) the effective quantum efficiency of PSII (ΦPSII). ΦPSII = (Fm′ − Fs)/fm′, where: Fm′ is light-adapted maximum fluorescence and Fs is the light-adapted steady-state fluorescence; 2) the electron transport rate of PSII (ETR). ETR = ΦPSII × PAR × 0.84 × 0.5 where the two numeric coefficients correct for light absorbance and the partitioning of light between the two photosystems, respectively: 3) the non-photochemical energy loss (quenching) of PSII largely corresponding to the light-dependent heat dissipation quantum efficiency of PSII (NPQ). NPQ = Fs/fm′ − Fs/fm.
Total leaf protein determination
Leaf protein contents were measured following the method described by Bradford (Bradford 1976). For protein extractions, 1 g of leaf was pulverized in a cold mortar by adding liquid nitrogen and 2 mL of cold extraction buffer (50 mM phosphate buffer pH 7.5). The homogenate was centrifuged at 12,000 x g for 15 min at 4 °C and the supernatant was used as an extract for protein content measurement. Total protein content was determined spectrophotometrically using Bradford - Solution for Protein Determination (AppliChem). The absorbance was measured at 595 nm. The calibration was made with bovine serum albumin (from 5 to 20 μg of BSA).
Detection of rubisco large sub-unit
Leaf tissues were homogenized using a cold mortar and pestle in the extraction buffer (Tris-HCl 50 mM pH. 7.5). The homogenates were centrifuged at 15,000×g at 4 °C for 15 min, and the supernatant was collected as a total protein extract. Equal protein loads (20 μg) were separated by 12.0% SDS-PAGE. Gels were stained by Comassie Brilliant Blue R-250 and the Large Sub-Unit of Rubisco (LSU) was marked in SDS-PAGE on the basis of molecular weight using molecular weight markers (BLUltra Prestained Protein Ladder, GeneDirex®). The molecular weight of the LSU band was determined previously (Tantray et al. 2020). Gel images were acquired using an Image Scanner (Brother MFC-J497DW). Digitized gel images were analyzed using the ImageJ software, to precisely measure LSU band area. Quantitative results were expressed as area % ± SD.
ABTS assay for the antioxidant capacity
The same extract used for determining total proteins (described in the previous section) was also used to determine antioxidant activity according to the method of (Re et al. 1999). This method is based on the decolorization of ABTS [2,29-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] following reduction of the radical cation, which was measured at 734 nm as a percent inhibition ((A0-A1)/A0 × 100, where A0 is the absorbance of the positive control and A1 is the absorbance of the sample). Ascorbic acid (Asa) was used as a standard antioxidant, at different concentrations (0–20 μM).
Analysis of fungi and yeasts in soil samples
At the end of the cultivation (10 days), a portion of soil was harvested from the pots, to test whether molds (fungi) and yeasts were potentially associated with the growth of plants with incremental percentages of CS. After isolation on Rose-Bengal selective agar culture medium by subculture of the microorganisms present in the test samples, DNA extraction was performed to amplify and subsequently sequence fungal genomes, targeting fungal 18S rRNA gene. Directly starting from the fungal colony, 2 g of mycelium from Rose-Bengal selective agar plates were aliquoted and centrifuged to recover DNA from the supernatant (Carraturo et al. 2018; Policastro et al. 2021). The supernatant was transferred into sterile 2 mL tubes containing 500 mg glass beads. CTAB extraction protocol (Corniello et al. 2022; Doyle 1991) was carried out to recover fungal DNA. The DNA samples were amplified with PCR, using a TECHNE Prime Thermal Cycler, for the characterization of fungi, employing ITS1 forward (5′-GGA AGT AAA AGT CGT AAC AAG G-3′ 5’-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 reverse (5′-TCC TCC GCT TAT TGA TAT GC-3′) primers (Biofab Research, Rome, Italy) complementary to ITS-5.8S rDNA region of the fungal 18S rRNA gene (700 bp) (Korabečná et al. 2003). Sequencing reactions were performed by an external service (Biofab Research, Rome, Italy); results were then interpreted using an editing tool, Chromas Lite v. 2.6.6 (Technelysium Pty Ltd., South Brisbane, Australia). The identification of the isolates was conducted using BLASTN ver. 2.2.29 (also referring to GenBank), selecting the identity holding the highest percentage of identity with a 95% cut-off and a minimum e-value lower than e − 460.
Statistical analysis
Data analyses were carried out using the Sigmaplot 14 software. Means ± standard deviations, or regression curves and 95% confidence intervals of 3 or more independent experiments are presented. Significant differences (p < 0.05) among the different growth condition were determined by one-way analysis of variance (ANOVA) with post-hoc Tukey HSD Test.
Results
Soil field capacity and water loss
CS addition increased the grams of water for grams of soil needed to reach FC (WFC) (Fig. 1). The WFC was comparable in Ctrl and 2% CS soil (respectively 1.06 ± 0.03 and 1.12 ± 0.01 g g−1) but significantly higher in 6% (1.37 ± 0.04 g g−1) and 10% (1.51 ± 0.11 g g−1) CS soils. A change in soil texture was also observed, as soils with added CS appeared lighter and more porous (Fig.1).
Amount of water needed to bring soil to field capacity (WFC) in the four different soils characterized by different concentrations of CS. Symbols represent means + SD (n = 6). Significant differences among the treatments were determined by one-way ANOVA with post-hoc Tukey HSD Test, and shown by different letters over the symbols (p < 0.05). At the bottom of the figure, pictures of the soil with different CS-enrichment (Ctrl, 2%, 6% and 10% CS) are shown
The water loss starting from water saturated-soils and until the Ctrl soil reached the complete dehydration (10 d) is shown in Fig. 2. Soil water content dropped significantly faster in Ctrl than in CS-enriched soils, and, after 10 d, CS- enriched soil held a growing percentage of water (23.07 ± 2.84% in 2% CS, 31.82 ± 1.20% in 6% CS, and 38.40 ± 11% in 10% CS. Water holding capacity of the CS-enriched soils was statistically improved over the entire experimental time (0–10 d) as shown by significant differences of regression curves and 95% confidence intervals with respect to Ctrl.
Water loss over the time of Ctrl soils and of soils enriched with different concentrations of CS. The soil dehydration was measured until the Ctrl soils was completely dry (0% water) after 10 d. Completely dry soil is identified by the red dashed line. Error bars represent SD (n = 6). The solid and dashed lines indicate the regression curve and 95% confidence intervals, respectively, with colors reflecting the different concentrations of CS
Plant growth
Both shoot and root length were significantly reduced in plants growing in soils enriched with 6% and 10% CS with respect to Ctrl (Table 1). Whereas seedlings cultivated on 2% CS showed a significant decrease only in the root length (−40% with respect to Ctrl). The shoot weight of seedlings grown with 6% and 10% CS was 30% and 45% less than in Ctrl, respectively. On the other hand, the root weight of plants grown with all CS-enriched soil was higher than in Ctrl. The total weight of the plants was not different in Ctrl and in plants grown with 6% and 10% CS. However, plants grown with 2% CS showed a significantly higher weight than in all other treatments.
Chlorophyll contents
The Chl a content did not undergo significant variations among treatments, whereas Chl b was significantly lower in plants grown with 2%, 6% or 10% CS with respect to Ctrl. The Chl a/Chl b ratio was significantly lower in plants grown with 6% or 10% CS than in Ctrl and plants grown with 2% SC (Table 2).
Fluorescence measurement
Chlorophyll fluorescence was used to assess in vivo the photosynthetic efficiency of plants under the different experimental conditions. The maximal photochemical efficiency in dark-adapted leaves (Fv/fm) was not different in Ctrl, 2% and 10% CS plants. In plants grown with 6% CS Fv/fm was significantly lower than all other conditions, albeit such a minor reduction did not indicate stress conditions (Fig. 3A). Plants grown under CS-enriched soil showed higher photochemical efficiency (ΦPSII, Fig. 3B), and electron transport rate (ETR, Fig. 3D), and lower non-photochemical quenching (NPQ, Fig.3C) than Ctrl.
Fluorescence parameters Fv/fm (panel A), ΦPSII (B), NPQ (C) and ETR (D) measured in vivo on barley seedlings grown in control (Ctrl) and CS-enriched (2%, 6%, and 10%) soils. Means and SD (n = 10) are shown. Significant differences were determined by one-way ANOVA with post-hoc Tukey HSD Test. In panel A, differences of means significant at P < 0.05 are shown by different letters. For the other panels, a complete statistical analysis of data is shown in Table S1
Rubisco LSU and protein contents
The amount of LSU of Rubisco decreased with increasing CS in the soil. The reduction in the LSU content with respect to Ctrl was 22, 31 and 38% respectively for plants grown with 2%, 6%, and 10% CS (Fig. 4).
A. Analysis of Rubisco LSU in Ctrl leaves and in leaves of plants grown with 2%, 6% or 10% CS. The area of Rubisco LSU bands from SDS-page electrophoresis were analyzed by Image J. In panel A, results from 3 LSU SDS-pages bands per treatment are expressed as % of band area with respect to Ctrl Rubisco (100%). Also shown are representative gels of the LSU bands in the four treatments. In panel B, the total protein contents were determined in extract from barley leaves. In both panel means are shown together with SD (n = 20). Differences among means were determined by one-way ANOVA with post-hoc Tukey HSD Test and significant differences (p < 0.05) are shown by different letters
The total protein content in plants grown with 10% CS was significant lower (−37%) than in Ctrl. Whereas, in plants grown with 2% and 6% CS there was no significant reduction of leaf total proteins with respect to controls (Fig. 4).
Antioxidant capacity
An increase in antioxidant capacity was recorded only in extracts from barley leaves grown on 10% CS. Whereas, growth on 2% and 6% CS yielded antioxidant capacities similar to that observed in Ctrl (Fig. 5).
Analysis of molds and yeasts in soil
The results (expressed as CFU/g) of the microbiological analyses looking for molds and yeasts in soils with incremental percentages of CS are showed in Table 3. After 10 d of plant cultivation, no fungi and yeasts were found in CS-enriched and Ctrl soils. Soil samples treated with increasing percentages of CS (2%, 6% and 10%) showed higher molds growth, especially at 10% CS (250 CFU/g). Different molds were found, depending on CS enrichment. In particular, Basipetospora chlamydospora was isolated from 2% CS, Penicillium oxalicum from 6% CS, and Aspergillus nidulans, Basipetospora chlamydospora and Emericella dentata from 10% CS-enriched soils.
Discussion
Today the reuse of waste products from agro-industrial processes has become an important goal of sustainable agriculture. The reason is twofold: to reduce the amount of waste, and to transform wasteful products in beneficial resources. Coffee Silverskin (CS) is the most abundant coffee roasting waste. For coffee producers, the management of this waste represents a cost, and its storage is also a risk as CS is highly flammable.
The objective of this research was to start evaluating the potential of CS as an agricultural soil fertilizer. Aspects concerning the soil, as well as growth, physiology and nutritional aspects of barley plants were considered. Coffee plants (roots, stems, leaves and beans) as well as coffee processed products (typically, exhausted coffee powder) are often contaminated by fungal infections, which represents a problem in the management of coffee value chain and processing (Pakshir et al. 2021; Viegas et al. 2017). We therefore paid attention to start experiments with uncontaminated CS from a variety of coffee (Uganda) which turns out to be devoid of microbial infection. However, different soil microbial communities were stimulated depending on the concentration of CS added to the soil. Basipetospora chlamydospora, predominantly found in 2% and 10% CS-enriched soils. This strain belongs to the order Microascales, in which it may represent a new genus (Barbosa et al. 2017), but still is a poorly studied fungus. Penicillium oxalicum, which was found in 6% CS- enriched soil, regulates the bioavailability of Pb in polluted soil (Xu et al. 2022) by Pb immobilization and reduction of the Pb exchangeable fraction (Hao et al. 2021; Long et al. 2018) P. oxalicum was also characterized as a Cr-tolerant microorganism, employed in bioremediation technologies aimed at the removal of toxic Cr(VI) by microbial reduction, which constitutes a promising approach to mitigate the ecotoxicological impact of Cr in the environment (Long et al. 2018). In addition, P. oxalicum is able to solubilize phosphorus by the secretion of organic acids containing oxalic, malic, and citric acids (Wang et al. 2021). Finally, Aspergillus nidulans and Emericella dentata were found in 10% CS-enriched soil. According to (Chen et al. 2016), these are the same organism, while (El-Sayed et al. 2019) considers them different organisms with taxonomical proximity and similar properties. The filamentous fungus A. nidulans commonly lives in the soil where it forms a hyphal network (Gerke et al. 2022; Riquelme et al. 2018), produces secondary metabolites (Gerke et al. 2022) and represents a suitable bio-sorbent for arsenic in contaminated soil (Maheswari and Murugesan 2009). Aspergillus nidulans, besides being one of the most abundant molds isolated from soil environment, was demonstrated surviving and handling Cd stress, through the up-regulation of the crpA Cu2+/Cd2+ pump gene, presumably depending on the metabolism of the AtfA gene, the principal transcription factor responsible of governing stress in this fungus (Emri et al. 2021). Emericella dentata produces meleagrin, a metabolite holding a potent in vitro cytotoxicity against the human cervix carcinoma cell line KB-3-1 (Hamed et al. 2021). While it is confirmed that enrichment of soils with coffee wastes (CS in our case) stimulates proliferation of soil microbial communities, none of the molds detected in soil supplemented with CS seems to have negative or toxic effects on plant growth. CS rather seems to be colonized by fungal species helping with the removal of soil contaminants and can be therefore considered as a substrate of potential interest for bioremediation processes.
The impact of CS on the physical properties of soils was also investigated. One of the main properties of a soil intended for agriculture is its field capacity, which is the maximum water retention power. Good field capacity ensures plants effortless and prolonged water supply. Soluble fibers are known to have water retaining properties (Bobková et al. 2022; Nolasco et al. 2022). About 8% of the CS is made of soluble fibers (Borrelli et al. 2004; Costa et al. 2018; Jiménez-Zamora et al. 2015), which means that we enriched by ~1.6, 4.8 and 8 mg/g the soluble fibers of soils containing 2%, 6%, and 10% CS, respectively. This might have significantly implemented the capacity of water retention by CS-enriched soils (Fig. 2) improving water content at field capacity (Fig. 1). However, when the ability to retain water over time was evaluated, it emerged that CS-enriched soils retain water longer than Ctrl soil. The soil that better manages water loss is the one added with 2% CS, as in this case FC was reached with the same amount of water as in Ctrl, but water was retained better than in Ctrl, over time. In a context of climate change and increasing shortage of water for agriculture, the use of moderate additions of CS may therefore help improving water management in soils, and the efficiency with which plants use this resource.
It is well known that the chemical and biological properties of soil influence plant growth and development. We have shown before that CS addition may improve water retention in soils. High soil colonization by microorganisms may also stimulate the release of plant exudates such as sugars, organic acids, amino acids or other components involved in the communication between roots and microorganisms (Prisa 2023). Microbial species can also influence plant nutrition by helping the acquisition of nutrients from soils (Prisa 2023).
Indeed, CS enrichment induced clear changes in barley plant growth. Plants grown on 6% and 10% CS were shorter both aboveground and belowground and showed reduced shoot weight compared to Ctrl. On the other hand, all plants grown with different CS enrichment showed an increase in root weight compared to Ctrl. Bashan and de-Bashan (2010) showed that soil microorganisms can produce bioactive molecules, such as phytohormones able to improve root thickening. Thus, the observed increased root weight of plants growing in CS-enriched soil might be related to the microbial stimulation. However, water availability might also have induced shorter and ticker roots with the same carbon investment otherwise dedicated to root elongation (Song et al. 2023). Interestingly, plants grown at 2% CS did not show the inhibitory effect on growth, except for a moderate reduction of root length. These plants weighted more than all other treatments at the end of the experiment which may indicate such a moderate CS concentration as the most effective to be used for fertilization purposes.
We investigated whether impairments of photosynthetic efficiency could explain the reduced growth of plants under moderate or high CS soil enrichment, as well as the improved growth of plants on 2% CS. The maximal quantum efficiency of PSII (Fv/Fm) was comparable in all plants, which indicates absence of photo-inhibitory conditions in all treatments (Maxwell and Johnson 2000). However, higher ɸPSII and ETR calculated at different PAR intensities indicate better photochemical efficiency in seedlings grown in CS-enriched soil than in Ctrl. Consistently, lower NPQ indicates a lower need of heat dissipation when plants are grown with CS (Maxwell and Johnson 2000, and references cited therein). On the other hand, our results showed a general decrease of Rubisco LSU levels in plants grown with CS, which seems to be dependent on CS concentration. In barley leaves grown with 10% CS, Rubisco LSU was approximately 40% than in Ctrl, and mirrored by a similar reduction of total proteins. The latter observation was not surprising as Rubisco makes about 50% of the foliar proteins. Thus, the reduction of growth observed in plants with CS-enriched soil may be caused by a lower biochemical efficiency, whereas the photochemical efficiency is preserved, and even stimulated. We do not know why plants grown with CS invest less on Rubisco and, in general, on leaf proteins. However, Chl b was also largely depleted in plants grown in CS-enriched soil, again with a strong CS-dependent gradient. This may have impaired Rubisco quantity and might also have a negative effect on the photochemistry of photosynthesis. The large increase of antioxidant capacity in 10% CS in fact suggests activation of mechanisms scavenging excess of reactive oxygen species formed when photosynthesis cannot use efficiently the electron transport rate. Chl b mainly populates light harvesting complexes (LHC) of the photosystem antennae, and the Chl a/Chl b ratio is commonly used as an indicator of antenna size. Antenna size decreases when Chl b content is reduced, for example due to a decreased activity of chlorophyll synthase enzyme or a nitrogen deficiency in the soil (Wang et al. 2021) influencing the efficiency of energy transfer to the chlorophylls of the reaction center (Melis 2009). However, antenna size reduction does not negatively influence photosynthesis as long as the maximal quantum yield of PSII remains unaffected and may even lead to improved efficiency of the remaining reaction centers in the photosystems (Gu et al. 2017; Kirst et al. 2017; Sakowska et al. 2018; Salbitani et al. 2020), which may explain why we observed improved photochemistry by chlorophyll fluorescence measurements in CS-enriched plants. Moreover, it should be considered that chlorophyll fluorescence only records active photosystems whereas does not identify photosystems that are not active anymore.
Perhaps reduction of Chl b and Rubisco LSU are related to the increase of soil microbial activity in CS-enriched plants, which may use in particular nitrogen to the detriment of Rubisco and leaf proteins. CS-enriched soils also have a high content of cellulose, hemicellulose and lignin which reaches over 75% of the dry biomass (Bobková et al. 2022). Thus, CS addition could temporarily alter the optimal proportion between carbon and nitrogen, causing nutritional constraints and stunted growth in plants. Indeed, addition of low amount of CS (2%) did not cause dramatic microbiological and chemical-physical changes to the soil, and also did not affect foliar protein content while improving water retention, photochemical efficiency, and total plant growth.
Conclusion
In conclusion, our results suggest that addition of a moderate concentration of CS might be the best to implement short-term fertilization effects, positively influencing plant growth. It is not fully clear why such a low addition of CS produced the best results. More efficient photosynthesis is often but not always accompanied by greater plant growth. In 2% CS-enriched plants our results indicate that PSII is fully functional, and that electron transport on the thylakoid membranes and protective dissipative mechanisms (non-photochemical quenching) are efficient, while CS does not positively influence protein content and higher CS enrichment are always detrimental to proteins and Rubisco, thus probably impairing photosynthesis. The results of this study showed that the use of CS, at low percentage, can represent a valid opportunity as an agricultural fertilizer both to dispose of the waste product in a sustainable way but also to obtain more productive and resource efficient plants. CS can be considered a natural and organic fertilizer, and its use on agricultural soils can represent a valid alternative for sustainable and eco-friendly disposal. However, as with all fertilizers, optimal use concentrations should be considered. Indeed, our results demonstrate that CS improves the soil water retention, the development of microorganism exploitable in bioremediation and, at low concentrations (CS 2% w/w), also improves the photosynthetic performance and growth of barley plants.
Data availability
Data will be made available on request.
References
Al-Zubade A, Phillips T, Williams MA, Jacobsen K, Van Sanford D (2021) Impact of nitrogen rate in conventional and organic production systems on yield and bread baking quality of soft red winter wheat. Agronomy 11:1683. https://doi.org/10.3390/agronomy11091683
Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C3 cereals: what should we breed for? Ann Bot 89 Spec No:925–940. https://doi.org/10.1093/aob/mcf049
Baik B-K, Ullrich SE (2008) Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci 48:233–242. https://doi.org/10.1016/j.jcs.2008.02.002
Barbosa RN, Leong SL, Vinnere-Pettersson O, Chen AJ, Souza-Motta CM, Frisvad JC, Samson RA, Oliveira NT, Houbraken J (2017) Phylogenetic analysis of Monascus and new species from honey, pollen and nests of stingless bees. Stud Mycol 86:29–51. https://doi.org/10.1016/j.simyco.2017.04.001
Bashan Y, de-Bashan LE (2010) Chapter two - how the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. In: Sparks DL (ed) Advances in agronomy. Academic Press, pp 77–136. https://doi.org/10.1016/S0065-2113(10)08002-8
Bessada S, Alves R, Costa A, Nunes M, Oliveira M (2018) Coffea canephora silverskin from different geographical origins: a comparative study. Sci Total Environ 645:1021–1028. https://doi.org/10.1016/j.scitotenv.2018.07.201
Bobková A, Poláková K, Demianová A, Belej Ľ, Bobko M, Jurčaga L, Gálik B, Novotná I, Iriondo-DeHond A, Castillo MDD (2022) Comparative analysis of selected chemical parameters of Coffea arabica, from cascara to silverskin. Foods Basel Switz 11:1082. https://doi.org/10.3390/foods11081082
Borrelli RC, Esposito F, Napolitano A, Ritieni A, Fogliano V (2004) Characterization of a new potential functional ingredient: coffee silverskin. J Agric Food Chem 52:1338–1343. https://doi.org/10.1021/jf034974x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Carfagna S, Vona V, Di Martino V, Esposito S, Rigano C (2011) Nitrogen assimilation and cysteine biosynthesis in barley: evidence for root Sulphur assimilation upon recovery from N deprivation. Environ Exp Bot 71(1):18–24. https://doi.org/10.1016/j.envexpbot.2010.10.008
Carraturo F, De Castro O, Troisi J, De Luca A, Masucci A, Cennamo P, Trifuoggi M, Aliberti F, Guida M (2018) Comparative assessment of the quality of commercial black and green tea using microbiology analyses. BMC Microbiol 18:4. https://doi.org/10.1186/s12866-017-1142-z
Chen W, Zhou P, Zhang M, Zhu Y, Wang X, Luo X, Bao Z, Yu L (2016) Transcriptome analysis reveals that up-regulation of the fatty acid synthase gene promotes the accumulation of docosahexaenoic acid in Schizochytrium sp. S056 when glycerol is used. Algal Res 15:83–92. https://doi.org/10.1016/j.algal.2016.02.007
Corniello A, Guida M, Stellato L, Trifuoggi M, Carraturo F, Del Gaudio E, Del Giudice C, Forte G, Giarra A, Iorio M, Marzaioli F, Toscanesi M (2022) Hydrochemical, isotopic and microbiota characterization of telese mineral waters (southern Italy). Environ Geochem Health 44:1949–1970. https://doi.org/10.1007/s10653-021-00806-4
Costa ASG, Alves RC, Vinha AF, Costa E, Costa CSG, Nunes MA, Almeida AA, Santos-Silva A, Oliveira MBPP (2018) Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem 267:28–35. https://doi.org/10.1016/j.foodchem.2017.03.106
Daneri-Castro SN, Svensson B, Roberts TH (2016) Barley germination: Spatio-temporal considerations for designing and interpreting ‘omics’ experiments. J Cereal Sci 70:29–37. https://doi.org/10.1016/j.jcs.2016.05.012
Doyle J (1991) DNA protocols for plants. In: Hewitt GM, Johnston AWB, Young JPW (eds) Molecular techniques in taxonomy, NATO ASI series. Springer, Berlin, pp 283–293. https://doi.org/10.1007/978-3-642-83962-7_18
El-Sayed ASA, Shindia AA, AbouZaid AA, Yassin AM, Ali GS, Sitohy MZ (2019) Biochemical characterization of peptidylarginine deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzym Microb Technol 124:41–53. https://doi.org/10.1016/j.enzmictec.2019.02.004
Emri T, Gila B, Antal K, Fekete F, Moon H, Yu J-H, Pócsi I (2021) AtfA-independent adaptation to the toxic heavy metal cadmium in Aspergillus nidulans. Microorganisms 9:1433. https://doi.org/10.3390/microorganisms9071433
European Commission (2020) https://ec.europa.eu/food/farm2fork_en
Francesca S, Najai S, Rong Z, Guillaume D, Cedric C, Carl-Otto O, Barone A, Rigano MM (2022) Phenotyping to dissect the biostimulant action of a protein hydrolysate in tomato plants under combined abiotic stress. Plant Physiol Biochem 179:32–43
Gerke J, Köhler AM, Wennrich J-P, Große V, Shao L, Heinrich AK, Bode HB, Chen W, Surup F, Braus GH (2022) Biosynthesis of antibacterial Iron-chelating Tropolones in Aspergillus nidulans as response to Glycopeptide-producing Streptomycetes. Front Fungal Biol 2. https://doi.org/10.3389/ffunb.2021.777474
Gu J, Zhou Z, Li Z, Chen Y, Wang Z, Zhang H (2017) Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Res. 200. https://doi.org/10.1016/j.fcr.2016.10.008
Hamed A, Abdel-Razek AS, Araby M, Abu-Elghait M, El-Hosari DG, Frese M, Soliman HSM, Stammler HG, Sewald N, Shaaban M (2021) Meleagrin from marine fungus Emericella dentata Nq45: crystal structure and diverse biological activity studies. Nat Prod Res 35:3830–3838. https://doi.org/10.1080/14786419.2020.1741583
Hao S, Tian J, Liu X, Wang P, Liu Y, Deng S, Zhang D (2021) Combined effects of Penicillium oxalicum and tricalcium phosphate on lead immobilization: performance, mechanisms and stabilities. Ecotoxicol Environ Saf 227:112880. https://doi.org/10.1016/j.ecoenv.2021.112880
Hejna A (2021) Coffee Silverskin as a potential bio-based antioxidant for polymer materials: brief review. Proceedings 69:20. https://doi.org/10.3390/CGPM2020-07220
International Coffee Organization-(ICO) (2023) Monthly coffee market report - October 2023. http://www.ico.org
Jiang J, Han J, Zhang X (2020) Nonhalogenated aromatic DBPs in drinking water chlorination: a gap between NOM and halogenated aromatic DBPs. Environ Sci Technol 54:1646–1656. https://doi.org/10.1021/acs.est.9b06403
Jiménez-Zamora A, Pastoriza S, Rufián-Henares JA (2015) Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT - Food Sci Technol 61:12–18. https://doi.org/10.1016/j.lwt.2014.11.031
Kirst H, Gabilly ST, Niyogi KK, Lemaux PG, Melis A (2017) Photosynthetic antenna engineering to improve crop yields. Planta 245:1009–1020. https://doi.org/10.1007/s00425-017-2659-y
Korabečná M, Liška V, Fajfrlík K (2003) PrimersITS1, ITS2 andITS4 detect the intraspecies variability in the internal transcribed spacers and 5.8S rRNA gene region in clinical isolates of fungi. Folia Microbiol. (Praha) 48:233–238. https://doi.org/10.1007/BF02930961
Long B, Ye B, Liu Q, Zhang S, Ye J, Zou L, Shi J (2018) Characterization of Penicillium oxalicum SL2 isolated from indoor air and its application to the removal of hexavalent chromium. PLoS One 13:e0191484. https://doi.org/10.1371/journal.pone.0191484
Lorbeer L, Schwarz S, Franke H, Lachenmeier DW (2022) Toxicological assessment of roasted coffee silver skin (Testa of Coffea sp.) as novel food ingredient. Molecules 27:6839. https://doi.org/10.3390/molecules27206839
Ludwig IA, Clifford MN, Lean MEJ, Ashihara H, Crozier A (2014) Coffee: biochemistry and potential impact on health. Food Funct 5:1695–1717. https://doi.org/10.1039/c4fo00042k
Lukinac J, Jukić M (2022) Barley in the production of cereal-based products. Plants 11:3519. https://doi.org/10.3390/plants11243519
Maheswari S, Murugesan AG (2009) Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ Technol 30:921–926. https://doi.org/10.1080/09593330902971279
Martuscelli M, Esposito L, Di Mattia CD, Ricci A, Mastrocola D (2021) Characterization of coffee silver skin as potential food-safe ingredient. Foods 10:1367. https://doi.org/10.3390/foods10061367
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280. https://doi.org/10.1016/j.plantsci.2009.06.005
Nolasco A, Squillante J, Velotto S, D’Auria G, Ferranti P, Mamone G, Errico ME, Avolio R, Castaldo R, Cirillo T, Esposito F (2022) Valorization of coffee industry wastes: comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J Clean Prod 370:133520. https://doi.org/10.1016/j.jclepro.2022.133520
Overturf E, Pezzutto S, Boschiero M, Ravasio N, Monegato A (2021) The CirCo (circular coffee) project: a case study on valorization of coffee Silverskin in the context of circular economy in Italy. Sustainability 13:9069. https://doi.org/10.3390/su13169069
Pakshir K, Dehghani A, Nouraei H, Zareshahrabadi Z, Zomorodian K (2021) Evaluation of fungal contamination and ochratoxin A detection in different types of coffee by HPLC-based method. J Clin Lab Anal 35:e24001. https://doi.org/10.1002/jcla.24001
Picca G, Plaza C, Madejón E, Panettieri M (2023) Compositing of coffee silverskin with carbon rich materials leads to high quality soil amendments. Waste Biomass Valor 14:297–307. https://doi.org/10.1007/s12649-022-01879-7
Policastro G, Carraturo F, Compagnone M, Giugliano M, Guida M, Luongo V, Napolitano R, Fabbricino M (2021) A preliminary study on a novel bioaugmentation technique enhancing lactic acid production by mixed cultures fermentation. Bioresour Technol 340:125595. https://doi.org/10.1016/j.biortech.2021.125595
Pourfarzad A, Mahdavian-Mehr H, Sedaghat N (2013) Coffee silverskin as a source of dietary fiber in bread-making: optimization of chemical treatment using response surface methodology. LWT Food Sci Technol 50:599–606. https://doi.org/10.1016/j.lwt.2012.08.001
Prisa D (2023) Role of microorganisms in communication between soil and plants. Karbala Int J Mod Sci 9. https://doi.org/10.33640/2405-609X.3287
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R (2018) Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol Mol Biol Rev MMBR 82:e00068–e00017. https://doi.org/10.1128/MMBR.00068-17
Rouphael Y, Colla G (2020) Editorial: Biostimulants in Agriculture. Front Plant Sci 11:40. https://doi.org/10.3389/fpls.2020.00040
Sakowska K, Alberti G, Genesio L, Peressotti A, Delle Vedove G, Gianelle D, Colombo R, Rodeghiero M, Panigada C, Juszczak R, Celesti M, Rossini M, Haworth M, Campbell BW, Mevy J-P, Vescovo L, Cendrero-Mateo MP, Rascher U, Miglietta F (2018) Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ 41:1427–1437. https://doi.org/10.1111/pce.13180
Salbitani G, Bolinesi F, Affuso M, Carraturo F, Mangoni O, Carfagna S (2020) Rapid and positive effect of bicarbonate addition on growth and photosynthetic efficiency of the green microalgae Chlorella Sorokiniana (Chlorophyta, Trebouxiophyceae). Appl Sci 10:4515
Salbitani G, Del Prete F, Carfagna S, Sansone G, Barone CMA (2021) Enhancement of pigments production by Nannochloropsis oculata cells in response to bicarbonate supply. Sustainability 13:11904. https://doi.org/10.3390/su132111904
Song Z, Zonta F, Ogorek LLP, Bastegaard VK, Herzog M, Pellegrini E, Pedersen O (2023) The quantitative importance of key root traits for radial water loss under low water potential. Plant Soil 482:567–584. https://doi.org/10.1007/s11104-022-05711-y
Srivastav AL, Patel N, Chaudhary VK (2020) Disinfection by-products in drinking water: occurrence, toxicity and abatement. Environ Pollut 267:115474. https://doi.org/10.1016/j.envpol.2020.115474
Tantray AY, Bashir SS, Ahmad A (2020) Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and rubisco efficiency of rice. Physiol Mol Biol Plants 26:83–94. https://doi.org/10.1007/s12298-019-00721-0
Toschi TG, Cardenia V, Bonaga G, Mandrioli M, Rodriguez-Estrada MT (2014) Coffee silverskin: characterization, possible uses, and safety aspects. J Agric Food Chem 62:10836–10844. https://doi.org/10.1021/jf503200z
Viegas C, Pacífico C, Faria T, de Oliveira AC, Caetano LA, Carolino E, Gomes AQ, Viegas S (2017) Fungal contamination in green coffee beans samples: a public health concern. J Toxicol Environ Health A 80:719–728. https://doi.org/10.1080/15287394.2017.1286927
Wang J, Zhao Y-G, Maqbool F (2021) Capability of Penicillium oxalicum y2 to release phosphate from different insoluble phosphorus sources and soil. Folia Microbiol (Praha) 66:69–77. https://doi.org/10.1007/s12223-020-00822-4
Xu Q, Jiang X, Tong J, Wu H, Luo Y, Shi J (2022) Penicillium oxalicum SL2 as a sustainable option to mitigate the accumulation of Pb in rice (Oryza sativa L.). Sci Total Environ 823:153769. https://doi.org/10.1016/j.scitotenv.2022.153769
Zhang Y, Zhao S, Liu S, Peng J, Zhang H, Zhao Q, Zheng L, Chen Y, Shen Z, Xu X, Chen C (2022) Enhancing the phytoremediation of heavy metals by combining Hyperaccumulator and heavy metal-resistant plant growth-promoting bacteria. Front Plant Sci 13. https://doi.org/10.3389/fpls.2022.912350
Acknowledgments
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) – MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 – D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Author information
Authors and Affiliations
Contributions
Conceptualization: G.S. and S.C.; Data curation: G.S., F.C., S.C.; Methodology: G.S., M.C., R.B., R.S., F.C., A.N., T.B., M.G.; Supervision F.L.; Writing - original draft: G.S. and S.C.; Writing - review & editing: G.S., F.L., S.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: W Richard Whalley.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salbitani, G., Chianese, M.R., Bossa, R. et al. Cultivation of barley seedlings in a coffee silverskin-enriched soil: effects in plants and in soil. Plant Soil 498, 199–211 (2024). https://doi.org/10.1007/s11104-023-06428-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06428-2