Abstract
Background
The present study aimed at determining whether, net of motor confounders, neuropsychological features affect functional independence (FI) in activities of daily living (ADLs) in non-demented amyotrophic lateral sclerosis (ALS) patients.
Methods
N = 88 ALS patients without frontotemporal dementia were assessed for FI—Katz’s Basic ADL Scale (BADL) and Lawton-Brody’s Instrumental ADL Scale (IADL)—, cognition—Edinburgh Cognitive and Behavioural ALS Screen (ECAS)—and behaviour—Beaumont Behavioural Inventory and Dimensional Apathy Scale. The association between cognitive and behavioural measures and BADL/IADL scores was assessed by covarying for demographics, anxiety and depression levels, disease duration and motor confounders—i.e. ALS Functional Rating Scale-Revised (ALSFRS-R) scores, progression rate and both King’s and Milano-Torino stages.
Results
Higher scores on the ECAS-Language were associated with higher IADL scores (p = 0.005), whilst higher apathetic features—as measured by the Dimensional Apathy Scale (DAS)—were inversely related to the BADL (p = 0.003). Whilst IADL scores were related to all ECAS-Language tasks, the DAS-Initiation was the only subscale associated with BADL scores. Patients with abnormal ECAS-Language (p = 0.023) and DAS (p = 0.008) scores were more functionally dependent than those without.
Discussion
Among non-motor features, language changes and apathetic features detrimentally affect FI in non-demented ALS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Frontotemporal-spectrum disorders (FTSDs) are acknowledged to detrimentally affect survival in non-demented amyotrophic lateral sclerosis (ALS) patients [1] by interfering with decision-making and adherence within care settings [2, 3].
However, little is known on the extent to which neuropsychological features impact on patients’ functional independence (FI) in daily living—likely due to their physical disabilities representing a major confounder to the study of such a matter [4, 5]. Only two reports have indeed to this day addressed this topic—the first, by Mioshi et al. [4], showing that FI was dependent on both motor and behavioural features, and the second, by Kapustin et al. [5], failing to detect an association between cognitive/behavioural features and FI net of ALS severity. However, these studies either preceded the availability of [4], or did not employ [5], ALS-specific cognitive/behavioural measures [6]. Moreover, the only study [5] having explored the association between FI and a performance-based measure of cognition did not provide single domain-level information.
The above being said, assessing how neuropsychological features impact FI in both basic and instrumental activities of daily living (ADL) in this population is prognostically pivotal, as it would shed further light on the ecological relevance of FTSDs in ALS besides their already acknowledged impact on survival [1, 2]. Hence, by employing a detailed and comprehensive set of ALS-specific cognitive and behavioural measures, the present study aimed at determining whether, net of motor confounders, FTSDs affect FI in non-demented ALS patients.
Methods
Participants
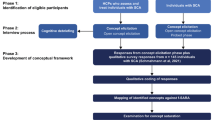
Eighty-eight ALS patients [7] consecutively referred to IRCCS Istituto Auxologico Italiano, Milano, Italy, between 2020 and 2023 were recruited. Patients did not present with (1) a co-morbid diagnosis of frontotemporal dementia (FTD) [8, 9], (2) ALS-unrelated neurological/psychiatric disorders, (3) severe/unstable general-medical conditions and (4) uncorrected sensory deficits.
Materials
FI was assessed via the Basic Activities of Daily Living Scale (BADL) by Katz et al. [10]—ranging 0–6 and assessing basic ADL—and the Instrumental Activities of Daily Living Scale (IADL) by Lawton and Brody [11]—ranging 0–8 and assessing instrumental ADL. Whenever at least one item on the IADL was not applicable, a proportion out of the applicable maximum was computed so that patients’ scores could be comparable among each other. Such “adjusted” IADL scores were computed by multiplying by 8 individual IADL scores weighted on their applicable maximum. The result of this computation was then rounded up or down the nearest integer if the first decimal digit was ≥ 0.50. For example, given an applicable maximum of 7 (i.e. one item not being applicable) and an actual score of 5, the “adjusted” IADL score—computed as (5/7)*8—is equal to 5.71—and thus 6 when rounded up. This adjustment was performed for 45 patients.
Cognition was assessed via the cognitive section of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) [12]—comprising 5 performance-based subscales tapping on Language (ECAS-L; range = 0–28), Fluency (ECAS-F; range = 0–24), Executive functioning (ECAS-EF; range = 0–48), Memory (ECAS-M; range = 0–24) and Visuospatial abilities (ECAS-VS; range = 0–12)—whilst behaviour with the Beaumont Behavioural Inventory (BBI) [13]—a 41-item, caregiver-report questionnaire covering the full spectrum of ALS patients’ behavioural phenotype (range = 0–123)—and the Dimensional Apathy Scale (DAS) [14]—a 24-item, self-report questionnaire assessing both cognitive and behavioural apathetic features (range = 0–72) and comprising three subscale tapping on dysexecutive features (DAS-Executive), affective disintegration (DAS-Emotional) and reduced cognitive/behavioural initiation (DAS-Initiation). Additionally, anxiety and depression levels were assessed via the State- and Trait-Anxiety Inventory-Form Y (STAI-Y1/-Y2) [15] and the Beck Depression Inventory (BDI) [16], respectively.
Motor status was assessed via the ALS Functional Rating Scale-Revised (ALSFRS-R) [17], progression rate (ΔFS) was computed according to Kimura et al. [18]—i.e. as (48-ALSFRS-R)/disease duration in months—and disease stage was retrieved based on both King’s [19] and Milano-Torino (MiToS) [20] systems.
Statistics
Both BADL and IADL scores were featured by a moderate ceiling effect and thus did not distribute normally—as indexed by an excessive, negative skewness value (i.e. >|1|) [21], a significant Shapiro–Wilk’s statistics (p < 0.001) and visual abnormalities within its histogram and Q-Q plot. Hence, non-parametric techniques and generalized linear models were employed for testing associations and predictions of interest, respectively.
First, the association between the BADL/IADL scores and both cognitive (i.e. ECAS-L, -F, -EF, -M and -VS scores) and behavioural measures (i.e. BBI and DAS scores) was preliminarily explored via Bonferroni-corrected Spearman’s coefficients that partialled out demographics—i.e. sex, age and education—, disease duration (in months), motor status (i.e. ALSFRS-R and ΔFS scores, King’s and MiToS stages) and psychopathological features (i.e. STAI-Y1/-Y2 and BDI scores).
Then, those cognitive and behavioural variables that proved to be significantly related to BADL/IADL scores within these correlational analyses were entered, along with the abovementioned covariates, into a negative binomial regression (NBR) which addressed, as the outcome, a reversed score on the BADL and IADL (rBADL; rIADL)—computed by subtracting from the theoretical maximum (i.e. 6 and 8, respectively) the actual score on the scale at hand. Such an expedient has been employed in order for BADL/IADL scores to adhere to the underlying count-like, and thus right-skewed, distribution which is modelled by the NBR [22]—by, at the same time, not undermining its original metric (since rBADL/rIADL scores reflect the degree of dependence). Within these NBRs, collinearity was diagnosed in the presence of a variance inflation factor (VIF) > 10 and of a tolerance index (TI) < 0.10.
Analyses were run via IBM® SPSS® Statistics (IBM Corp., 2021) and jamovi 2.3 (the jamovi project, 2022). Within the correlational set, missing data points were excluded pairwise, whilst, within the NBRs, a listwise deletion procedure was applied.
Results
Table 1 summarizes patients’ background and clinical measures.
The results of correlational analyses are reported in Table 2. Net of demographics, disease duration, motor status and psychopathological features, the only associations surviving Bonferroni’s correction (i.e. αadjusted = 0.007) were those between (1) IADL scores and the ECAS-L (positive coefficient) and (2) BADL scores and the DAS (negative coefficient).
Accordingly, two NBRs were run: the first, addressing rIADL scores as the outcome and the ECAS-L as the predictor; the second, addressing rBADL scores as the outcome and the DAS as the predictor. The results of these models are shown in Table 3. No collinear regressors were detected within either the model addressing the ECAS-L (VIF ≤ 5.43; TI ≥ 0.18) or that addressing the DAS (VIF ≤ 5.55; TI ≥ 0.18). Both models were in agreement with the previous correlational analyses—with the ECAS-L and the DAS being predictive of rIADL and rBADL scores, respectively. As to covariates, within both models, lower age and lower ALSFRS-R scores were also associated a higher degree of functional dependence; additionally, within the model addressing the rBADL, female sex, higher MiToS scores and lower STAI-Y2 scores were associated with a poorer FI.
Notably, when re-running the same NBRs by substituting the ECAS-L and the DAS with their respective below- vs. above-cutoff scores [12, 14], such predictors retained their significance (Table 4)—with patients performing defectively on the ECAS-L (23.9%) being more functionally dependent on the rIADL (M = 2.24; SE = 0.60) than those performing within the normal range (M = 1.08; SE = 0.24), and patients with an above-cutoff DAS score (27%) being more functionally dependent on the rBADL (M = 1.12; SE = 0.43) than those with a below-cutoff score on this scale (M = 0.38; SE = 0.11).
In order to exploratively appraise which task(s) of the ECAS-L were associated with the IADL, a Spearman’s correlational set was addressed, which covaried for demographics (i.e. age, education and sex), disease duration and motor status (i.e. ALSFRS-R, ΔFS, King’s and MiToS scores). Such Spearman’s coefficients revealed that IADL score were related to all ECAS-L tasks—i.e. Naming (rs(88) = 0.25; p = 0.025), Comprehension (rs(88) = 0.26; p = 0.021) and Spelling (rs(88) = 0.23; p = 0.041).
Consistently, the same explorative set of Spearman’s coefficient were run between BADL scores and DAS subscales—by nevertheless also adding STAI-Y1/-Y2 and BDI scores as covariates, given the relevance of such psychiatric features to apathy [23]. These analyses revealed that the BADL was selectively associated with the DAS-Initiation (rs(80) = − 0.28; p = 0.019), whilst not with DAS-Executive (rs(80) = − 0.17; p = 0.159) and -Emotional scores (rs(80) = − 0.11; p = 0.354).
Discussion
The present study provides relevant insights into the role of FTSDs towards FI in non-demented ALS patients, suggesting that language deficits and apathetic features detrimentally affect everyday-life functioning in this population. More specifically, language dysfunctions—as assessed by the dedicated ECAS subscale—herewith proved to be linked to worse IADL scores, whilst a diminished cognitive-behavioural initiation—as assessed by the Initiation subscale of the DAS—was related to worse BADL scores.
To the best of the authors’ knowledge, this is the first study addressing such a topic by (1) simultaneously encompassing a wide range of both motor and non-motor variables and (2) employing ALS-specific measures of both cognition and behaviour—this granting a sufficiently high level of generalizability to the findings herewith reported.
Overall, the current study aligns with Mioshi et al.’s [4] findings as to the fact that not only motor, but also extra-motor features affect FI in ALS—thus not supporting Kapustin et al.’s [5] recent investigation, where ALSFRS-R scores happened to be the only predictor of FI measures in this population.
The present finding of apathetic features being linked to a lower degree of FI in ALS is consistent with Mioshi et al.’s [4] results—where the Motivation subscale of the Cambridge Behavioural Inventory-Revised [24] proved to be, along with its Abnormal behaviour subscale and ALSFRS-R-Spinal scores, a significant predictor of the Disability Assessment of Dementia [25]. In addition, the current report sheds a further light on the link between apathy and FI in ALS, suggesting that a specific component of this syndrome—i.e. a reduced cognitive-behavioural initiation—impacts on basic ADL in this population. Whilst such a finding is unprecedented within the literature concerning apathy in ALS [25], a cumulative effect of initiation deficits to motor disabilities might be postulated in order to account for it: otherwise said, in patients with a greater decrease in goal-directed activity, motor disabilities might have impacted even more on their FI in basic ADL. Whilst such a hypothesis is of course speculative and thus needs to be further tested, this report still happens align with and add up to the current knowledge on the adverse effect that apathy exerts on ALS patients’ prognosis [25]. Moreover, it is worth mentioning that the current results are also consistent with the literature revealing a detrimental effect of apathetic features towards FI in order neurodegenerative disorders—such as Alzheimer’s, Parkinson’s and Huntington’s disease, as well as frontotemporal degeneration [27,28,29,30].
Relevantly, and unprecedentedly when compared to previous studies on the topic [4, 5], this report suggest that FI in instrumental ADLs also depends on cognition, and specifically on language, in ALS. Interestingly, such a finding happens to be unparalleled by the relevant literature addressing other neurodegenerative conditions—such as Alzheimer’s, Parkinson’s and Huntington’s disease, as well as frontotemporal degeneration [31,32,33,34,35,36,37,38]—, where behavioural disturbances, as well as deficits within the executive domain, are rather associated with impaired FI. Nevertheless, this result is per se not surprising—since language impairment (LI) might have easily undermined patients’ communicative and comprehension skills, thus in turn reducing their FI in cognitive-driven ADL (such as those tapped onto by the IADL). After all, the impact of LI on FI in primary language disorders—such as post-stroke aphasia [39] or primary progressive aphasia (PPA) [40]—is widely acknowledged: hence, it is reasonably expected that also mild-to-moderate language deficits, albeit not resulting in a full-blown aphasic syndrome, detrimentally affect FI in instrumental ADL in this population too.
The present study also sheds a light, for the first time, on the ecological relevance of LI in this population. LI within the spectrum of PPA [41,42,43,44] occurs in ≈23% of non-demented ALS patients [45], with its detection being also sufficient to classify patients as cognitively impaired according to Strong et al.’s [1] revised criteria for FTSDs in ALS. Moreover, the incidence of LI in this population has been reported to increase over time [46]. Adding up to such stances, findings herewith reported highlight that LI in non-demented ALS patients is relevant not only at a diagnostic level, but also from a prognostic perspective. Further research on the prognostic role of LI in this population is thus worthwhile, also within the longitudinal dimension [47]—and, thus, by advisably addressing technology-aided language assessment procedures that, as fully overcoming motor limitations, are feasible across all disease stages [48,49,50].
Finally, as far as the covariates entered within the NBRs, this study disclosed a number of incidental findings that are worth a tentative explanation. First, younger age unexpectedly proved to be inversely related to both BADL and IADL scores. Such a result is highly controversial, given that older age has been typically linked to a greater degree of functional dependence [51]. However, both the BADL and the IADL have been reported to be possibly biased by age [52,53,54,55]—with some items being more likely to be endorsed by younger individuals and others by older ones. Hence, it is likely that the present findings regarding age might be measurement-specific, rather than reflecting an actual association between this demographic and FI in ALS. A similar explanation might be applicable to the finding of female sex being associated with lower BADL scores, since sex biases in the ADL scales herewith employed have been described as well—i.e. some items being more likely to be endorsed by males and others by females [52, 53, 55]. As to the association between higher STAI-Y2 scores and higher BADL scores, a mediating role of apathetic features might be advanced. In fact, an inverse association has been recently reported in this population between anxiety—albeit a state-level—and apathy—albeit as far as emotional disintegration is concerned [23]. Since apathy has been herewith found detrimentally affect FI, it might be postulated that patients with a greater degree of apathetic features, and thus of functional dependence, were at the same time features by lower arousal—and thus anxiety—levels. Nevertheless, such an explanation is merely speculative and not empirically supported on the basis of the current results. Whilst it would be far beyond the aim of this study to explore the interplay between apathy, anxiety and FI in ALS, this matter might explored in future studies.
This study is of course not free of limitations. First, FI was herewith operationalized via aspecific scales that are known to tap on cognitive-driven ADL to a limited extent [56]. Hence, future studies on the association between FTSDs and FI in this population should address ADL scales which heavily load on cognitive functioning—such as the Amsterdam IADL Questionnaire© [57], which has shown excellent clinimetrics and feasibility in neurodegenerative disorders [57,58,59,60,61]. Second, within the present study, cognition has been evaluated by means of a screening test—i.e. the ECAS: although this test has been thoroughly shown to be clinimetrically sound and feasible within the Italian scenario [12, 47, 63, 64], it is undoubtedly advisable that future studies further investigate the interplay between FTSDs and FI in this population by employing domain-/function-specific, second-level tests. Relatedly, it has to be mentioned that the ECAS-L has been criticized as not being able to fully cover the spectrum of ALS patients’ language phenotypes [45, 62, 63]: thus, future investigations should aim at replicating—or disconfirming—the present findings by employing a detailed and extensive set of second-level language measures. Finally, behavioural features were herewith assessed via both a caregiver-report—i.e. the BBI—and a self-report scale—i.e. the DAS. Such a discrepancy related to the source of information on patients’ behavioural status might have altered, at least to some extent, the current findings. It is thus advisable that future investigations on the topic address a consistent source of information.
In conclusion, among FTSDs, language changes might affect instrumental ADL in non-demented ALS patients, whilst apathetic features—and, more specifically, a diminished cognitive-behavioural initiation—might impact on basic ADL in this population.
Data availability
Datasets associated with the present study have been stored on an online repository (https://doi.org/10.5281/zenodo.8384038) and are available upon reasonable request of interested researchers.
References
Strong MJ, Abrahams S, Goldstein LH et al (2017) Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18:153–174
Huynh W, Ahmed R, Mahoney CJ et al (2020) The impact of cognitive and behavioral impairment in amyotrophic lateral sclerosis. Expert Rev Neurother 20:281–293
Poletti B, Carelli L, Lunetta C, Ticozzi N, Silani V (2020) Advance care planning and mental capacity in ALS: a current challenge for an unsolved matter. Neurol Sci 41:2997–2998
Mioshi E, Lillo P, Kiernan M, Hodges J (2012) Activities of daily living in motor neuron disease: role of behavioural and motor changes. J Clin Neurosci 19:552–556
Kapustin D, Zarei S, Wang W, et al. (2023) Neuropsychiatric symptom burden across neurodegenerative disorders and its association with function. Can J Psychiatry. 07067437221147443.
Gosselt IK, Nijboer TC, Van Es MA (2020) An overview of screening instruments for cognition and behavior in patients with ALS: selecting the appropriate tool for clinical practice. Amyotroph Lateral Scler Frontotemporal Degener 21:324–336
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Gorno-Tempini ML, Hillis AE, Weintraub S et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW (1963) Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Poletti B, Solca F, Carelli L et al (2016) The validation of the Italian Edinburgh cognitive and behavioural ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener 16(17):489–498
Iazzolino B, Pain D, Laura P et al (2022) Italian adaptation of the Beaumont Behavioral Inventory (BBI): psychometric properties and clinical usability. Amyotroph Lateral Scler Frontotemporal Degener 23:81–86
Santangelo G, Siciliano M, Trojano L et al (2017) Apathy in amyotrophic lateral sclerosis: insights from dimensional apathy scale. Amyotroph Lateral Scler Frontotemporal Degener 18:434–442
Siciliano M, Trojano L, Trojsi F, Monsurro MR, Tedeschi G, Santangelo G (2019) Assessing anxiety and its correlates in amyotrophic lateral sclerosis: the state-trait anxiety inventory. Muscle Nerve 60:47–55
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169:13–21
Kimura FC, Fujimura C, Ishida S et al (2006) Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 66:265–267
Roche JC, Rojas-Garcia R, Scott KM et al (2012) A proposed staging system for amyotrophic lateral sclerosis. Brain 135:847–852
Chiò A, Hammond ER, Mora G, Bonito V, Filippini G (2015) Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 86:38–44
Kim HY (2013) Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod 38:52–54
Green JA (2021) Too many zeros and/or highly skewed? A tutorial on modelling health behaviour as count data with Poisson and negative binomial regression. Health Psychol Behav Med 9:436–455
Poletti B, Solca F, Maffi S et al (2022) Semiology and determinants of apathy across neurodegenerative motor disorders: a comparison between amyotrophic lateral sclerosis, Parkinson’s and Huntington’s disease. Front Aging Neurosci 14:1031908
Wear HJ, Wedderburn CJ, Mioshi E et al (2008) The Cambridge behavioural inventory revised. Dem Neuropsychol 2:102–107
Gelinas I, Gauthier L, McIntyre M, Gauthier S (1999) Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 53:471–481
Kutlubaev MA, Caga J, Xu Y, Areprintseva DK, Pervushina EV, Kiernan MC (2023) Apathy in amyotrophic lateral sclerosis: systematic review and meta-analysis of frequency, correlates, and outcomes. Amyotroph Lateral Scler Frontotemporal Degener 24:14–23
Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL (2003) Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry 11:214–221
Laatu S, Karrasch M, Martikainen K, Marttila R (2013) Apathy is associated with activities of daily living ability in Parkinson’s disease. Dement Geriatr Cogn Disord 35:249–255
Yassuda MS, da Silva TB, O’Connor CM et al (2018) Apathy and functional disability in behavioral variant frontotemporal dementia. Neurol Clin Pract 8:120–128
Fritz NE, Boileau NR, Stout JC et al (2018) Relationships among apathy, health-related quality of life, and function in Huntington’s disease. J Neuropsychiatry Clin Neurosci 30:194–201
Martyr A, Clare L (2012) Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dementia Geriatr Cogn Disord 33(2–3):189–203
Lechowski L, Dieudonne B, Tortrat D et al (2003) Role of behavioural disturbance in the loss of autonomy for activities of daily living in Alzheimer patients. Int J Geriatr Psychiatry 18:977–982
Moheb N, Mendez MF, Kremen SA, Teng E (2017) Executive dysfunction and behavioral symptoms are associated with deficits in instrumental activities of daily living in frontotemporal dementia. Dement Geriatr Cognit Disord 43(1–2):89–99
Murley AG, Rouse MA, Coyle-Gilchrist IT et al (2021) Predicting loss of independence and mortality in frontotemporal lobar degeneration syndromes. J Neurol Neurosurg Psychiatry 92:737–744
Hamilton JM, Salmon DP, Corey-Bloom J et al (2003) Behavioural abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 74:120–122
Ross CA, Pantelyat A, Kogan J, Brandt J (2014) Determinants of functional disability in Huntington’s disease: role of cognitive and motor dysfunction. Mov Disord 29:1351–1358
Bronnick K, Ehrt U, Emre M et al (2006) Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry 77:1136–1142
Leroi I, Ahearn DJ, Andrews M, McDonald KR, Byrne EJ, Burns A (2011) Behavioural disorders, disability and quality of life in Parkinson’s disease. Age Ageing 40:614–621
Flowers HL, Skoretz SA, Silver FL et al (2016) Poststroke aphasia frequency, recovery, and outcomes: a systematic review and meta-analysis. Arch Phys Med Rehabil 97:2188–2201
O’Connor CM, Ahmed S, Mioshi E (2014) Functional disability in primary progressive aphasia. Aphasiology 28:1131–1149
Pinto-Grau M, Hardiman O, Pender N (2018) The study of language in the amyotrophic lateral sclerosis-frontotemporal spectrum disorder: a systematic review of findings and new perspectives. Neuropsychol Rev 28:251–268
Sbrollini B, Preti AN, Zago S, Papagno C, Appollonio IM, Aiello EN (2022) Language impairment in motor neuron disease phenotypes different from classical amyotrophic lateral sclerosis: a review. Aphasiology 36:1373–1396
Aiello EN, Feroldi S, Preti AN, Zago S, Appollonio IM (2022) Dysgraphic features in motor neuron disease: a review. Aphasiology 36:1249–1274
Aiello EN, Feroldi S, De Luca G, et al. (2022) Primary progressive aphasia and motor neuron disease: a review. Front Aging Neurosci. 1080.
Solca F, Aiello EN, Torre S et al (2023) Prevalence and determinants of language impairment in non-demented amyotrophic lateral sclerosis patients. Eur J Neurol 30:606–611
Consonni M, Dalla Bella E, Bersano E, Lauria G (2021) Cognitive and behavioural impairment in amyotrophic lateral sclerosis: a landmark of the disease? A mini review of longitudinal studies. Neurosci Lett 754:135898
Poletti B, Solca F, Carelli L et al (2018) Cognitive-behavioral longitudinal assessment in ALS: the Italian Edinburgh cognitive and behavioral ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener 19:387–395
Poletti B, Carelli L, Solca F et al (2017) An eye-tracker controlled cognitive battery: overcoming verbal-motor limitations in ALS. J Neurol 264:1136–1145
Poletti B, Carelli L, Solca F et al (2017) An eye-tracking controlled neuropsychological battery for cognitive assessment in neurological diseases. Neurol Sci 38:595–603
Poletti B, Carelli L, Faini A et al (2018) The Arrows and Colors Cognitive Test (ACCT): a new verbal-motor free cognitive measure for executive functions in ALS. Plos One 13:e0200953
Liao WL, Chang YH (2020) Age trajectories of disability in instrumental activities of daily living and disability-free life expectancy among middle-aged and older adults in Taiwan: an 11-year longitudinal study. BMC Geriatr 20:530
Fleishman JA, Spector WD, Altman BM (2002) Impact of differential item functioning on age and gender differences in functional disability. J Gerontol B Psychol Sci Soc Sci 57:S275–S284
Niti M, Ng TP, Chiam PC, Kua EH (2007) Item response bias was present in instrumental activity of daily living scale in Asian older adults. J Clin Epidemiol 60:366–374
LaPlante MP (2010) The classic measure of disability in activities of daily living is biased by age but an expanded IADL/ADL measure is not. J Gerontol B Psychol Sci Soc Sci 65:720–732
Jang SN, Kawachi I (2019) Why do older Korean adults respond differently to activities of daily living and instrumental activities of daily living? A differential item functioning analysis. Ann Geriatr Med Res 23:197
Hancock P, Larner AJ (2007) The diagnosis of dementia: diagnostic accuracy of an instrument measuring activities of daily living in a clinic-based population. Dement Geriatr Cognit Disord 23:133–139
Sikkes SA, de Lange-de Klerk ES, Pijnenburg YA et al (2012) A new informant-based questionnaire for instrumental activities of daily living in dementia. Alzheimers Dement 8:536–543
Sikkes SA, Knol DL, Pijnenburg YA, De Lange-de Klerk ES, Uitdehaag BM, Scheltens P (2013) Validation of the Amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology 41:35–41
Sikkes SA, Pijnenburg YA, Knol DL, de Lange-de Klerk ES, Scheltens P, Uitdehaag BM (2013) Assessment of instrumental activities of daily living in dementia: diagnostic value of the Amsterdam Instrumental Activities of Daily Living Questionnaire. J Geriatr Psychiatry Neurol 26:244–250
Koster N, Knol DL, Uitdehaag BM, Scheltens P, Sikkes SA (2015) The sensitivity to change over time of the Amsterdam IADL Questionnaire©. Alzheimers Dement 11:1231–1240
Jutten RJ, Peeters CF, Leijdesdorff SM et al (2017) Detecting functional decline from normal aging to dementia: development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement 8:26–35
McMillan CT, Wuu J, Rascovsky K et al (2022) Defining cognitive impairment in amyotrophic lateral sclerosis: an evaluation of empirical approaches. Amyotroph Lateral Scler Frontotemporal Degener 23:517–526
Aiello EN, Iazzolino B, Pain D et al (2022) The diagnostic value of the Italian version of the Edinburgh cognitive and behavioral ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener 23:527–531
Aiello EN, Solca F, Torre S et al (2023) Reliable change indices for the Italian Edinburgh cognitive and behavioral ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener 24:339–342
Acknowledgements
The authors are thankful to patients and their caregivers. The authors acknowledge the ERN Euro-NMD for support.
Funding
This research was funded by the Italian Ministry of Health to IRCCS Istituto Auxologico Italiano (Ricerca Corrente, project 23C302).
Author information
Authors and Affiliations
Contributions
ENA: conceptualization, analyses, drafting, revision; FeSo, ST, FG, FrSc, MO, EC, AM: data collection, revision; MM: revision; CM, AD, FV, VS: resources, revision; BP, NT: resources, drafting, revision.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committees of IRCCS Istituto Auxologico Italiano (I.D.: 2013_06_25).
Informed consent
Participants provided informed consent.
Conflict of interest
VS received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, Liquidweb S.r.l., Novartis Pharma AG and Zambon and receives or has received research supports from the Italian Ministry of Health, AriSLA and E-Rare Joint Transnational Call. He is in the Editorial Board of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, European Neurology, American Journal of Neurodegenerative Diseases, Frontiers in Neurology and Exploration of Neuroprotective Therapy. BP received compensation for consulting services and/or speaking activities from Liquidweb S.r.l. She is Associate Editor for Frontiers in Neuroscience. NT received compensation for consulting services from Amylyx Pharmaceuticals and Zambon Biotech SA. He is Associate Editor for Frontiers in Aging Neuroscience.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aiello, E.N., Solca, F., Torre, S. et al. Frontotemporal-spectrum disorders and functional independence in non-demented ALS patients. Neurol Sci 45, 1087–1095 (2024). https://doi.org/10.1007/s10072-023-07074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07074-3