Abstract
Background
The hearing success of patients with bimodal fitting, utilizing both a cochlear implant (CI) and a hearing aid (HA), varies considerably: While some patients benefit from bimodal CI and HA, others do not.
Objectives
This retrospective study aimed to investigate speech perception in bimodally fitted patients and compare it with the cochlear coverage (CC).
Methods
The CC was calculated with the OTOPLAN software, measuring the cochlear duct length on temporal bone CT scans of 39 patients retrospectively. The patients were categorized into two groups: CC ≤ 65% (CC500) and CC > 65% (CC600). Monaural speech intelligibility for monosyllables at a sound pressure level (SPL) of 65 dB in a free-field setting was assessed before and after CI at various time points. The two groups, one with preoperative HA and one with postoperative CI, were compared. Additionally, speech intelligibility was correlated with CC in the entire cohort before CI and at the last available follow-up (last observation time, LOT).
Results
Overall, there was no significant difference in speech intelligibility between CC500 and CC600 patients, with both groups demonstrating a consistent improvement after implantation. While CC600 patients tended to exhibit earlier improvement in speech intelligibility, CC500 patients showed a slower initial improvement within the first 3 months but demonstrated a steeper learning curve thereafter. At LOT, the two patient groups converged, with no significant differences in expected speech intelligibility. There was no significant relationship between unimodal/unilateral free-field speech intelligibility and CC. Interestingly, patients with a CC of 70–75% achieved the highest speech intelligibility.
Conclusion
Despite of the lack of a significant correlation between CC and speech perception, patients appeared to reach their maximum in unimodal/unilateral speech perception primarily at a coverage level of 70–75%. Nevertheless, further investigation is warranted, as CC500 was associated with shorter cochlear duct length, and different types of electrodes were used in both groups.
Similar content being viewed by others
Physioanatomical characteristics
Paramount for the success of cochlear implants (CI) is the individualized care of each patient The CI team takes into account the diverse (patho)physioanatomical characteristics of each patient, including inner ear malformations [1], residual hearing [2, 3], prevention of vertigo [4], and the wide range of cochlear duct lengths (CDL; [5,6,7]). Different CI manufacturers offer a selection of electrodes with carrying lengths and locations within the cochlea [8,9,10,11]. The modality should also be considered when fitting both ears.
Modality categories
A substantial proportion of CI recipients fall into one of the following five categories: (1) Patients with single-sided deafness (SSD) who have normal hearing in one ear and a CI in the other ear [12,13,14]. (2) Bimodally fitted patients with asymmetric hearing, fitted with CI in one ear and a contralateral hearing aid (HA) in the better, not CI-indicated ear [12]. (3) Bimodally fitted patients who meet the indication criteria for a CI on both ears but are fitted with CI in the worse and HA in the better-hearing ear [15]. (4) Patients with electroacoustic stimulation (EAS) in one ear and a HA in the other ear. These patients have good low-frequency residual hearing and receive an HA integrated into the CI audio processor, which allows simultaneous electrical and acoustic stimulation from the CI processor itself [3, 16]. (5) Bilaterally deaf patients fitted with bilateral CIs [17].
Variability in binaural integration
However, within these categories there are considerable differences in the degree of binaural integration. Some patients with bilateral fittings experience substantial benefit from binaural hearing, while others experience minimal to no benefit. Disadvantages, such as binaural interference, are also possible. Several individual characteristics contribute to this variability, including residual hearing preservation [18], cortical plasticity, duration of deafness [19], differing processing times of CI and HA [20, 21], frequency discrepancy between CI and HA ear [22, 23], and differences in automatic gain control between CI and HA [24]. Bimodal interference is observed in some patients, who report better hearing when using only one ear [25, 26]. Another factor that may influence the success of bimodal fitting is the cochlear coverage (CC) provided by the electrode array. The question is whether a monaural CI with a larger CC can offer better low-frequency hearing and, thus, avoid interference with the contralateral side supplied by the HA. Therefore, the aim of the study was to investigate monaural speech perception in bimodally fitted patients with CI and HA with respect to the CC.
Materials and methods
Patients
A retrospective, single-center analysis was performed comprising a total of 39 bimodally fitted patients. Pre- and postoperative audiometric data, as well as the radiological Stenverʼs view to assess CI electrode placement (complete full insertion in all patients), were available for evaluation. Patients were implanted with either FLEX28 (28 mm, active stimulation length 23.1 mm) or FLEXSOFT electrodes (31.5 mm, active stimulation length 26.4 mm) from MED-EL (Innsbruck, Austria; [27]).
Cochlear coverage
The CC was determined in all patients using the OTOPLAN software (CAScination AG, Bern, Switzerland, version 2) based on CT images (CE certification number: G1 17 10 95657 003). During preoperative planning, the software enables measurement of the cochlea using Digital Imaging and Communications in Medicine® (DICOM®) datasets, to determine the insertion depth of the CI electrode array and the CC [28].
All DICOM® datasets were initially reviewed for image quality and temporal bone malformations by a radiologist experienced in temporal bone anatomy before being uploaded into the software. Exclusion criteria were cochlear malformations, CT slice thickness of ≥ 0.7 mm, and datasets that could not be transferred to the OTOPLAN software for technical reasons.
The cochlea was measured preoperatively in three planes using the software described in detail elsewhere [7]. In brief, the software calculated the length of the cochlear duct using an elliptical-circular approximation (ECA) based on the measured values of “A-value” (maximum distance between the round window and the contralateral wall), “B-value” (distance between the walls of the cochlea perpendicular to the line of the A‑value), and “height” (distance perpendicular to the basal turn of the cochlea to the apex; [29]). The CC was then determined computationally using the expected angular insertion depth (AID) from the preoperative CT dataset for the selected electrodes, and the frequency-location mapping in the cochlea was estimated using the Greenwood function based on selected electrodes [29]. A CC of 100% corresponded to 2.5 turns of the cochlea, resulting in an AID of 900° [30]. All measurements were performed by two independent examiners blinded to each other’s measurements and electrode information, and the resulting measurement results were averaged.
For further analysis of CC, participants were divided into two comparator groups of approximately equal size, regardless of electrode type. One group had a CC of ≤ 65% (mean AID of this cohort: 498.6°; designated CC500) and the other group a CC of > 65% (mean AID of this cohort: 591.1°; designated CC600).
Audiometric data
To perform hearing threshold audiometry, sinusoidal tones standardized according to German DIN EN ISO 8253 were presented successively in different frequencies between 0.250 and 8 kHz. This was done under both air conduction and free-field conditions in an audiometrically isolated booth. If necessary, the non-tested ear was additionally masked with noise according to the guidelines of the Comité Consultatif International Télégraphique et Téléphonique (CCITT) to prevent cross-over hearing on the opposite ear. The tones were presented through headphones unaided for air conduction; for free field they were presented through a loudspeaker to assess separately the aided hearing threshold with HA/CI (inflation curve) side by side. The resulting pre- and postoperative thresholds, measured in decibel hearing level (dB HL), were compared and documented. For the postoperative values, the most recent hearing status data from the patient’s medical record, referred to as the last available observation time (LOT) for follow-up, was used.
Speech intelligibility, measured with the German Freiburg Speech Test standardized according to German DIN 45621‑1 and DIN 45626‑1 at a sound pressure level (SPL) of 65 dB [31], was obtained retrospectively from electronic medical records.
Monaural monosyllable aided speech perception was assessed in the implanted ear, preoperatively with HA and postoperatively with CI (‑audio processor). Postoperative testing was performed at the time of initial fitting (IF), 1 month (1M), 3 months (3M), and 1 year (12M) after initial fitting, and at LOT.
Statistical analysis
Statistical analysis of the data was performed using Microsoft Excel (Microsoft, Redmond, WA, USA, version 2110) and the Statistical Package for Social Sciences (SPSS) software (IBM, Armonk, NY, USA, version 28).
For normally distributed data, the unpaired samples t test was used to compare the means of CC and several cochlear morphology parameters (CDL, A‑value, B‑value, and AID), and speech intelligibility between the CC500 and CC600 groups. In the case of non-normal distributions, the Mann–Whitney U test was performed to compare the median values of cochlear height and speech intelligibility between the CC500 and CC600 groups. The correlation between speech perception for monosyllables and CC was also examined using the Pearson correlation coefficient. The significance level was set at 0.05.
Results
Demographics
The median age of the 39 patients enrolled in the study at the time of implantation was 65 years (15–90 years). Of these patients, 27 received a FLEX28 electrode and 12 a FLEXSOFT electrode. Table 1 provides an overview of the etiology of profound sensorineural hearing loss leading to deafness in the patient population.
Cochlear coverage
Of the 39 patients, 14 were in the CC500 group and 25 were in the CC600 group. The mean CC for the CC500 group was 60.6 ± 3.6%, while the CC for the CC group600 was 73.1 ± 5.4%. The mean CC for the entire cohort was 68.6 ± 7.7%. Table 2 shows additional parameters such as CDL, A and B values, height, and AID. The t tests revealed significant differences between the C500 and CC600 group for CC (t(37) = −8.61; p < 0.001), CDL (t(37) = 3.67; p = 0.001), A‑value (t(37) = 2.74; p = 0.009), B‑value (t(37) = 3.77; p = 0.001), and AID (t(37) = −5.96; p < 0.001). However, there was no significant difference regarding height (U = 116.50; Z = −1.72; p > 0.05).
Audiometric data
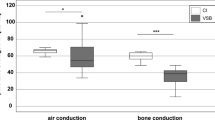
Audiometry was performed on 39 bimodally fitted patients using air conduction and free-field audiometry. Figure 1a shows the consistent decrease in air conduction hearing threshold for the implanted ear preoperatively without HA from 57 dB HL at 125 Hz to 115 dB HL at 8 kHz, with a pure tone average (PTA; 0.5; 1; 2; 4 kHz) of 87.4 dB HL. Similarly, preoperative air conduction for the nonimplanted contralateral ear showed a consistent decrease from 39 dB HL at 125 Hz to 92 dB HL at 8 kHz, resulting in a PTA of 59.3 dB HL (Fig. 1b).
For the implanted ear, the free-field hearing threshold showed a decreasing trend along with increasing frequency with HA preoperatively, ranging from 40 dB HL at 125 Hz to 75 dB HL at 8 kHz (PTA = 53.2 dB HL; Fig. 1c). With the CI at LOT, the hearing thresholds were similar across frequencies, ranging from 39 dB HL at 125 Hz to 37 dB HL at 8 kHz (PTA = 44.7 dB HL; Fig. 1c). The preoperative PTA for the HA-equipped contralateral ear averaged 49.9 dB HL, and the PTA at the LOT averaged 43.9 dB HL (Fig. 1d). Additional pure-tone audiometric data for the implanted ear at LOT are presented in Table 3.
Regarding the assessment for normality, all datasets, except for monosyllable intelligibility of the C600 group, displayed normality at the IF and at the 1M appointment (Shapiro–Wilk p < 0.001 and p = 0.02). The t tests and Mann–Whitney U test results revealed no significant differences in speech intelligibility between CC500 and CC600 at the observed time points. Figure 2, using box–whisker plots, illustrates a trend in which speech perception in the implanted ear initially appeared to deteriorate from 20% of the group median at the preoperative measurement to 0% at the IF, but showed steady improvement postoperatively with the CI at the subsequent measurement time points: 1M after IF (25%), 3M (30%), 12M (40%), and at LOT (60%). At IF, patients in the CC500 group initially appeared to perform better in speech perception (7.5%) than patients in the CC600 group (0%); however, this difference was not significant. However, at the 1M appointment, patients with CC500 showed a slight reduction in speech perception (20%) compared to those with CC600 (27.5%). At 3M, patients with CC500 (15%) appeared to have worse speech perception than those with CC600 (40%). However, between the 3 and 12M appointments, patients with CC500 (50%) appeared to have greater improvement in speech perception compared to those with CC600 (40%). At LOT, the two groups converged in speech perception. Additional speech perception data with the implanted ear at LOT are presented in Table 3.
Box–whisker plots of speech perception for monosyllables at 65 dB SPL in the groups of patients with cochlear coverage (CC) of CC ≤ 65% (CC500), CC > 65% (CC600), and the entire cohort (CC total = CC500 + CC600) at different observation times: preoperatively (pre-OP), initial fitting (IF), 1 month (1 Mon), 3 months (3 Mon), and 12 months (12 Mon) after IF, and at the last available observation time point (LOT). Outliers are marked with +
Correlation of cochlear coverage with speech perception
Correlation analysis revealed no significant correlation between total CC (total cohort of CC500 and CC600) and intelligibility at 65 dB SPL, neither preoperatively monaurally with HA (n = 14; r = −0.16; p > 0.05) nor postoperatively with a CI at LOT (n = 34; r = −0.09; p > 0.05). Figure 3 illustrates that patients with approximately 70–75% CC achieved maximum speech intelligibility within the entire cohort.
Discussion
The present study
The primary objective of this study was to investigate the correlation between CC in bimodally fitted patients (CI + contralateral HG) and monaural speech perception in the implanted ear.
No significant correlation between CC and monaural speech perception was observed at any time point for the entire group of patients.
Interestingly, contrary to our assumption that complete CC (CC = 100%, corresponding to an AID of 900° or an insertion of 2.5 turns) would result in better speech perception, we found a maximum in speech perception at approximately 70–75% CC. However, due to the large interindividual variability in speech perception, the low nonsignificant coefficients, and the small sample size, these results should be interpreted with caution. Future studies with a larger patient cohort are needed to validate the hypothesis of a maximum at 70–75% CC. It is also important to include patients with 50–60% CC and 80–90% CC, as they were underrepresented in this study. This may shed light on the possible nonlinear relationship between CC and speech perception. The effect of preoperative residual hearing on speech perception and its association with CC was not thoroughly investigated in this study. Further research is warranted to investigate the influence of residual hearing on speech perception outcomes. In addition, within-subject variability in speech intelligibility was observed at different test time points, which may be due to various psychological/behavioral factors such as motivation or concentration. These factors further complicate the interpretation of the results.
Nevertheless, our results show that the CC500 and CC600 groups converge at the LOT with similar medians and dispersions after different levels of speech perception with electrical stimulation by CI. It is worth noting that the shorter CC500 group initially required a longer learning period for speech perception.
Comparison with other studies
Previous studies have not reached a consensus on whether there is a relationship between CC of the electrode array and monaural speech perception with CI. For example, Doubi et al. [32] divided prelingually deafened children under 7 years of age into two groups and found no significant difference in speech perception, measured by the speech intelligibility rating test, between a group with CC < 85% and a group with CC ≥ 85% at 3 years postoperatively. They concluded that stimulation of the most apical region of the cochlea does not necessarily provide a benefit for speech perception. Other studies have used a metric comparable to CC, the angular insertion depth (AID), and examined its relationship to speech perception. The majority of these studies reported no correlation between speech perception and AID [19, 20, 25, 37, 42, 43]. Heutink et al. [33] conducted a comprehensive systematic review of previous studies and found no significant correlation in six out of seven studies [34,35,36,37,38,39]. However, other studies have shown a relationship between AID and speech perception: O’Connel et al. [40] observed a significant positive correlation in postlingually deafened adults, measured 12–16 months postoperatively, with a 0.6% increase in the English Consonant Nucleus Consonant (CNC) score at 60 dB (A) per 10° AID. Similar positive correlations were found by Canfarotta et al. [41] using the CNC score of adult unilateral CI recipients measured 12 months after IF and by Heutink et al. [42] using the Dutch Consonant–Vowel–Consonant test measured in adult CI users with at least 1 year of unilateral hearing experience. By contrast, Ketterer et al. [43] examined adult CI recipients with the Freiburg Monosyllabic Test at 65 dB SPL at regular intervals and found a significant negative relationship indicating a decrease in speech perception with increasing insertion depth. Although we did not find a significant relationship between insertion depth and monaural speech perception in our study, larger insertion depths (> 75%) appeared to have a negative effect on speech perception. This could be explained by the possibility that deeper insertions are more likely to damage any residual hearing that may be present. Ketterer et al. [43] attributed their results to cross-turn stimulation, which may occur with deeply inserted apical electrodes. However, deep insertion was underrepresented in the study by Ketterer et al. [43], with only approximately 2% of the ears included (10 out of 495) having a CC > 75%. In addition, better preoperative speech perception may lead to better postoperative speech perception with CI.
An advantage of electrical stimulation at the apex of the cochlea is improved perception of low frequencies, which are also particularly important for music perception. When listening to music, a mixed cohort of bilateral and SSD CI users with longer electrodes (31.5 mm) experienced better perception of lower frequencies compared to users with 7.5 mm shorter electrodes (24 mm) due to the more extensive apical stimulation [44]. This results in improved discrimination of sound quality for patients with long electrode arrays.
Speck et al. [38] conducted a study to investigate the effects of different electrode lengths (active stimulation length: 15.0 mm versus 19.1 mm versus 23.1 mm) on speech reception thresholds (SRT) in SSD patients. Assessment of SRT was made under two different noise conditions: speech and noise collocated at the front (S0N0) and speech on the implanted side with masking noise on the normal-hearing side. In both conditions, no significant difference in SRT was found between the electrode array lengths.
Deep insertion may provide a more natural hearing experience, especially for bimodal CI users with an HA on the contralateral side, as well as for SSD CI users, because the electrodes of the lower frequencies are closer to the apical spiral ganglion cells that correspond to these low frequencies according to Greenwood’s frequency-location mapping in the cochlea [30]. Presumably, this is the reason why patients with a deeper insertion in this study adapted more quickly to hearing with the CI and went through the learning process faster than those with a shallower insertion, because the sound field perceived over the CI was less pitch shifted. Whether this holds true for all postlingually deafened patients remains to be tested.
Practical conclusion
-
We did not find a significant correlation between monaural monosyllabic speech perception with a cochlear implant (CI) and the cochlear coverage (CC) provided by the CI electrode in bimodally (CI and hearing aid [HA]) fitted patients.
-
A trend was observed: speech perception increased with increasing CC, reaching a maximum at ca. 70–75% and then decreased with further increases in CC.
-
The lack of a significant relationship may be due to the large variability in a small patient population, resulting in insufficient statistical power, as well as the possibility of a nonlinear relationship between CC and speech perception. Linear Pearson correlation analysis was not suitable for assessing the possibility of a nonlinear relationship, partly due to the covariance of the electrode array (FLEX28, FLEXSOFT).
-
Patients with a greater insertion depth achieved faster learning success. Despite different CC500/CC600 mean values (59.2 ± 28.4%/47.1 ± 21.9%) and medians (65%/60%) of the CC, no significant difference in long-term speech perception was found.
-
In clinical practice, preoperative measurement of the cochlea and individualized electrode selection are beneficial to determine the most suitable electrode length for every patient.
-
A CC of 70–75% was identified as a good reference point for CC, but more research is needed in this area before a definitive recommendation can be made.
References
Sennaroglu L, Sarac S, Ergin T (2006) Surgical results of cochlear implantation in malformed cochlea. Otol Neurotol 27:615–623. https://doi.org/10.1097/01.mao.0000224090.94882.b4
Bruce IA, Todt I (2018) Hearing preservation cochlear implant surgery. Adv Otorhinolaryngol 81:66–73. https://doi.org/10.1159/000485544
Helbig S, Helbig M, Leinung M, Stöver T, Baumann U, Rader T (2015) Hearing preservation and improved speech perception with a flexible 28-mm electrode. Otol Neurotol 36:34–42. https://doi.org/10.1097/MAO.0000000000000614
Nordfalk KF, Rasmussen K, Hopp E, Bunne M, Silvola JT, Jablonski GE (2016) Insertion depth in cochlear implantation and outcome in residual hearing and vestibular function. Ear Hear 37:e129–37. https://doi.org/10.1097/AUD.0000000000000241
Erixon E, Rask-Andersen H (2013) How to predict cochlear length before cochlear implantation surgery. Acta Otolaryngol 133:1258–1265. https://doi.org/10.3109/00016489.2013.831475
Rask-Andersen H, Liu W, Erixon E, Kinnefors A, Pfaller K, Schrott-Fischer A, Glueckert R (2012) Human cochlea: anatomical characteristics and their relevance for cochlear implantation. Anat Rec (Hoboken) 295:1791–1811. https://doi.org/10.1002/ar.22599
Spiegel JL, Polterauer D, Hempel J‑M, Canis M, Spiro JE, Müller J (2022) Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet-based software. Eur Arch Otorhinolaryngol 279:1851–1861. https://doi.org/10.1007/s00405-021-06889-0
Battmer R‑D, Scholz S, Gazibegovic D, Ernst A, Seidl RO (2020) Comparison of a mid Scala and a perimodiolar electrode in adults: performance, impedances, and psychophysics. Otol Neurotol 41:467–475. https://doi.org/10.1097/MAO.0000000000002579
Dhanasingh A, Hochmair I (2021) Special electrodes for demanding cochlear conditions. Acta Otolaryngol 141:157–177. https://doi.org/10.1080/00016489.2021.1888506
MacPhail ME, Connell NT, Totten DJ, Gray MT, Pisoni D, Yates CW, Nelson RF (2022) Speech recognition outcomes in adults with slim straight and slim modiolar cochlear implant electrode arrays. Otolaryngol Head Neck Surg 166:943–950. https://doi.org/10.1177/01945998211036339
Mewes A, Brademann G, Hey M (2020) Comparison of perimodiolar electrodes: imaging and electrophysiological outcomes. Otol Neurotol 41:e934–e944. https://doi.org/10.1097/MAO.0000000000002790
Arndt S, Laszig R, Aschendorff A, Hassepass F, Beck R, Wesarg T (2017) Cochlea-Implantat-Versorgung von Patienten mit einseitiger Taubheit oder asymmetrischem Hörverlust. HNO 65:98–108. https://doi.org/10.1007/s00106-016-0297-5
Döge J, Baumann U, Weissgerber T, Rader T (2017) Single-sided deafness: impact of cochlear implantation on speech perception in complex noise and on auditory localization accuracy. Otol Neurotol 38:e563–e569. https://doi.org/10.1097/MAO.0000000000001520
Távora-Vieira D, Rajan G, van de Heyning P, Mertens G (2021) Quality standards for adult cochlear implantation in single-sided deafness and asymmetric hearing loss. B‑ENT 16:209–216. https://doi.org/10.5152/B-ENT.2021.20172
Cullington HE, Zeng F‑G (2011) Comparison of bimodal and bilateral cochlear implant users on speech recognition with competing talker, music perception, affective prosody discrimination, and talker identification. Ear Hear 32:16–30. https://doi.org/10.1097/AUD.0b013e3181edfbd2
von Ilberg CA, Baumann U, Kiefer J, Tillein J, Adunka OF (2011) Electric-acoustic stimulation of the auditory system: a review of the first decade. Audiol Neurootol 2:1–30. https://doi.org/10.1159/000327765
Rader T, Fastl H, Baumann U (2013) Speech perception with combined electric-acoustic stimulation and bilateral cochlear implants in a multisource noise field. Ear Hear 34:324–332. https://doi.org/10.1097/AUD.0b013e318272f189
Hoppe U, Hocke T, Digeser F (2018) Bimodal benefit for cochlear implant listeners with different grades of hearing loss in the opposite ear. Acta Otolaryngol 138:713–721. https://doi.org/10.1080/00016489.2018.1444281
Beyea JA, McMullen KP, Harris MS, Houston DM, Martin JM, Bolster VA, Adunka OF, Moberly AC (2016) Cochlear implants in adults: effects of age and duration of deafness on speech recognition. Otol Neurotol 37:1238–1245. https://doi.org/10.1097/MAO.0000000000001162
Zirn S, Arndt S, Aschendorff A, Wesarg T (2015) Interaural stimulation timing in single sided deaf cochlear implant users. Hear Res 328:148–156. https://doi.org/10.1016/j.heares.2015.08.010
Zirn S, Angermeier J, Arndt S, Aschendorff A, Wesarg T (2019) Reducing the device delay mismatch can improve sound localization in bimodal cochlear implant/hearing-aid users. Trends Hear 23:2331216519843876. https://doi.org/10.1177/2331216519843876
Bernstein JGW, Goupell MJ, Schuchman GI, Rivera AL, Brungart DS (2016) Having two ears facilitates the perceptual separation of concurrent talkers for bilateral and single-sided deaf cochlear Implantees. Ear Hear 37:289–302. https://doi.org/10.1097/AUD.0000000000000284
Rader T, Döge J, Adel Y, Weissgerber T, Baumann U (2016) Place dependent stimulation rates improve pitch perception in cochlear implantees with single-sided deafness. Hear Res 339:94–103. https://doi.org/10.1016/j.heares.2016.06.013
Spirrov D, Kludt E, Verschueren E, Büchner A, Francart T (2020) Effect of (Mis)matched compression speed on speech recognition in bimodal listeners. Trends Hear 24:2331216520948974. https://doi.org/10.1177/2331216520948974
Mok M, Grayden D, Dowell RC, Lawrence D (2006) Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Speech Lang Hear Res 49:338–351. https://doi.org/10.1044/1092-4388(2006/027)
Wess JM, Brungart DS, Bernstein JGW (2017) The effect of Interaural mismatches on contralateral unmasking with single-sided vocoders. Ear Hear 38:374–386. https://doi.org/10.1097/AUD.0000000000000374
MED EL Electronics Designed for atraumatic implantation providing superior hearing performance. https://s3.medel.com/pdf/21617.pdf. Accessed 01.12.2022
CAScination. https://www.cascination.com/en/otoplan. Accessed: 01.12.2022
Schurzig D, Timm ME, Batsoulis C, Salcher R, Sieber D, Jolly C, Lenarz T, Zoka-Assadi M (2018) A novel method for clinical cochlear duct length estimation toward patient-specific cochlear implant selection. OTO Open 2:2473974X18800238. https://doi.org/10.1177/2473974X18800238
Greenwood DD (1990) A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am 87:2592–2605. https://doi.org/10.1121/1.399052
Hahlbrock K‑H (1953) Über Sprachaudiometrie und neue Wörterteste. Arch Ohren Nasen Kehlkopfheilkd 162:394–431. https://doi.org/10.1007/BF02105664
Doubi A, Almuhawas F, Alzhrani F, Doubi M, Aljutaili H, Hagr A (2019) The effect of cochlear coverage on auditory and speech performance in cochlear implant patients. Otol Neurotol 40:602–607. https://doi.org/10.1097/MAO.0000000000002192
Heutink F, de Rijk SR, Verbist BM, Huinck WJ, Mylanus EAM (2019) Angular electrode insertion depth and speech perception in adults with a cochlear implant: a systematic review. Otol Neurotol 40:900–910. https://doi.org/10.1097/MAO.0000000000002298
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G et al (2013) Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 34:342–360. https://doi.org/10.1097/AUD.0b013e3182741aa7
de Seta D, Nguyen Y, Bonnard D, Ferrary E, Godey B, Bakhos D, Mondain M, Deguine O, Sterkers O, Bernardeschi D et al (2016) The role of electrode placement in bilateral simultaneously cochlear-implanted adult patients. Otolaryngol Head Neck Surg 155:485–493. https://doi.org/10.1177/0194599816645774
Hilly O, Smith L, Hwang E, Shipp D, Symons S, Nedzelski JM, Chen JM, Lin VYW (2016) Depth of cochlear implant array within the cochlea and performance outcome. Ann Otol Rhinol Laryngol 125:886–892. https://doi.org/10.1177/0003489416660111
Marrinan MS, Roland JT, Reitzen SD, Waltzman SB, Cohen LT, Cohen NL (2004) Degree of modiolar coiling, electrical thresholds, and speech perception after cochlear implantation. Otol Neurotol 25:290–294. https://doi.org/10.1097/00129492-200405000-00015
van der Beek FB, Boermans PPBM, Verbist BM, Briaire JJ, Frijns JHM (2005) Clinical evaluation of the Clarion CII HiFocus 1 with and without positioner. Ear Hear 26:577–592. https://doi.org/10.1097/01.aud.0000188116.30954.21
van der Marel KS, Briaire JJ, Verbist BM, Muurling TJ, Frijns JHM (2015) The influence of cochlear implant electrode position on performance. Audiol Neurootol 20:202–211. https://doi.org/10.1159/000377616
O’Connell BP, Cakir A, Hunter JB, Francis DO, Noble JH, Labadie RF, Zuniga G, Dawant BM, Rivas A, Wanna GB (2016) Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol 37:1016–1023. https://doi.org/10.1097/MAO.0000000000001125
Canfarotta MW, Dillon MT, Brown KD, Pillsbury HC, Dedmon MM, O’Connell BP, O’Connell BP (2022) Insertion depth and cochlear implant speech recognition outcomes: a comparative study of 28- and 31.5-mm lateral wall arrays. Otol Neurotol 43:183–189. https://doi.org/10.1097/MAO.0000000000003416
Heutink F, Verbist BM, van der Woude W‑J, Meulman TJ, Briaire JJ, Frijns JHM, Vart P, Huinck WJ, Mylanus EAM (2021) Factors influencing speech perception in adults with a cochlear implant. Ear Hear 42:949. https://doi.org/10.1097/AUD.0000000000000988
Ketterer MC, Aschendorff A, Arndt S, Beck R (2022) Electrode array design determines scalar position, dislocation rate and angle and postoperative speech perception. Eur Arch Otorhinolaryngol 279:4257–4267. https://doi.org/10.1007/s00405-021-07160-2
Roy AT, Penninger RT, Pearl MS, Wuerfel W, Jiradejvong P, Carver C, Buechner A, Limb CJ (2016) Deeper Cochlear Implant Electrode Insertion Angle Improves Detection of Musical Sound Quality Deterioration Related to Bass Frequency Removal. Otol Neurotol 37:146–151. https://doi.org/10.1097/MAO.0000000000000932
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T. Rader designed the study, wrote the paper, designed the graphics, and provided critical revisions. L. Schrank collected and analyzed the data wrote the paper, and provided critical revisions. J.L. Spiegel conceived of the study, collected the data, wrote the paper, and provided critical revisions. P. Nachtigäller wrote the paper, designed the graphics, and provided critical revisions. J.E. Spiro, J.-M. Hempel, M. Canis, and J. Mueller provided critical revision. All authors discussed the results and implications and provided input into the manuscript at all stages.
Corresponding author
Ethics declarations
Conflict of interest
J.L. Spiegel and T. Rader received educational support from MED-EL GmbH, Innsbruck, Austria and Cochlear Deutschland GmbH & Co KG, Hanover, Germany L. Schrank, P. Nachtigäller, J.E. Spiro, J.-M. Hempel, M. Canis and J. Mueller declare that they have no competing interests. The supplement containing this article is not sponsored by industry.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the responsible ethics committee of the Ludwig-Maximilians-University of Munich (reference 19-562) and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
The German version of this article can be found under https://doi.org/10.1007/s00106-023-01330-w.

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rader, T., Schrank, L., Spiegel, J.L. et al. Comparison of speech perception in bimodal cochlear implant patients with respect to the cochlear coverage. HNO 72 (Suppl 1), 17–24 (2024). https://doi.org/10.1007/s00106-023-01327-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00106-023-01327-5