Abstract
Purpose
To investigate prevalence and predictors of renal function variation in a multicenter cohort treated with radical nephroureterectomy (RNU) for upper tract urothelial carcinoma (UTUC).
Methods
Patients from 17 tertiary centers were included. Renal function variation was evaluated at postoperative day (POD)—1, 6 and 12 months. Timepoints differences were Δ1 = POD-1 eGFR − baseline eGFR; Δ2 = 6 months eGFR − POD-1 eGFR; Δ3 = 12 months eGFR − 6 months eGFR. We defined POD-1 acute kidney injury (AKI) as an increase in serum creatinine by ≥ 0.3 mg/dl or a 1.5 1.9-fold from baseline. Additionally, a cutoff of 60 ml/min in eGFR was considered to define renal function decline at 6 and 12 months. Logistic regression (LR) and linear mixed (LM) models were used to evaluate the association between clinical factors and eGFR decline and their interaction with follow-up.
Results
A total of 576 were included, of these 409(71.0%) and 403(70.0%) had an eGFR < 60 ml/min at 6 and 12 months, respectively, and 239(41.5%) developed POD-1 AKI. In multivariable LR analysis, age (Odds Ratio, OR 1.05, p < 0.001), male gender (OR 0.44, p = 0.003), POD-1 AKI (OR 2.88, p < 0.001) and preoperative eGFR < 60 ml/min (OR 7.58, p < 0.001) were predictors of renal function decline at 6 months. Age (OR 1.06, p < 0.001), coronary artery disease (OR 2.68, p = 0.007), POD-1 AKI (OR 1.83, p = 0.02), and preoperative eGFR < 60 ml/min (OR 7.80, p < 0.001) were predictors of renal function decline at 12 months. In LM models, age (p = 0.019), hydronephrosis (p < 0.001), POD-1 AKI (p < 0.001) and pT-stage (p = 0.001) influenced renal function variation (ß 9.2 ± 0.7, p < 0.001) during follow-up.
Conclusion
Age, preoperative eGFR and POD-1 AKI are independent predictors of 6 and 12 months renal function decline after RNU for UTUC.
Similar content being viewed by others
Introduction
Urothelial carcinoma (UC) is the sixth most common malignancy, and upper urinary tract UC (UTUC) accounts for 5–10% of cases [1]. UTUC has an estimated annual incidence of two cases per 100,000, rising in recent years as a result of enhanced detection and improved bladder cancer survival [2]. UTUC has high-mortality, with more than 150,000 deaths per year [1]. Radical nephroureterectomy (RNU), including removal of kidney, entire ureter, and bladder cuff, is the gold standard for high-risk localized UTUC. Low-risk disease, defined as the presence of unifocal, small (< 2 cm), low-grade and superficial tumor, is suitable for kidney-sparing approaches, which provide equal survival outcomes preserving renal function [3].
UTUC patients have an increased risk of chronic kidney disease(CKD) because of age, comorbidities, smoking exposure, and potential impairment of contralateral kidney due to diagnostic procedures or contralateral UTUC [4]. Previous studies showed that CKD may lead to worse overall and cancer-specific survival after treatment for renal cell carcinoma (RCC) as well as UTUC [5, 6]. Despite the benefit of adjuvant chemotherapy in prolonging survival [7] and reducing the risk of disease recurrence in locally advanced UTUC [8], 50% of patients are not eligible for platinum-based protocols due to postoperative renal failure [9, 10]. The identification of patients at risk of significant renal function decline may allow clinicians to better assess kidney-sparing rather than extirpative surgery. Additionally, it may help in developing more appropriate protective strategies such as neoadjuvant treatments or different adjuvant approaches and in a more adequate follow-up schedule for these patients [11, 12]. Clinical factors such as age, cardiovascular disease and low preoperative estimated glomerular filtration rate(eGFR) have been previously associated with renal function decline after RNU [13,14,15]. However, large-scale and contemporary analyses on predictors associated with renal function impairment are missing. No data have been reported in the recent literature on renal function variation (considering reduction and recovery) after RNU.
The aim of this study is to investigate the prevalence and predictors of renal function variation in a large multicenter cohort of patients who underwent RNU for UTUC.
Materials and methods
We enrolled 1979 patients from 17 urology tertiary centers affiliated with the Radical Nephroureterectomy Outcomes (RaNeO) Research Consortium, who underwent RNU for UTUC between 1994 and 2020. Only patients who had complete preoperative, postoperative and follow-up renal function data were included. Exclusion criteria consisted of previous or concurrent radical cystectomy, contralateral or metastatic UTUC, and previous renal parenchymal sparing surgery. All patients provided written informed consent for data collection and analysis.
Renal function was evaluated preoperatively, at postoperative day 1(POD-1), at 6 and 12 months. Estimated-GFR, according to Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) equation [16], was used. A cutoff of 60 ml/min in eGFR was considered to define renal function decline. POD-1 acute kidney injury(AKI) was defined as an increase in serum creatinine by ≥ 0.3 mg/dl or a 1.5–1.9-fold from baseline, according to the Acute Kidney Injury Network(AKIN) classification [17].
The following preoperative data were also retrospectively collected in all centers: gender, age at surgery, body mass index (BMI), American Society of Anesthesiologists(ASA) classification of physical status, and Eastern Cooperative Oncology Group(ECOG) score, comorbidities (presence of coronary artery disease[CAD]-, hypertension, hyperlipidemia, diabetes mellitus), smoking exposure, presence of ipsilateral hydronephrosis, preoperative albumin serum level, and preoperative hemoglobin serum level. Perioperative data included operative time, blood loss, intraoperative blood transfusions, POD-1 AKI, POD-1 hemoglobin serum level, postoperative chemotherapy, pathological TNM stage revised according to the AJCC classification system (8th edition) [18], tumor grade, and positive surgical margins(PSMs) rate.
Statistical analysis
Statistical analysis consisted of three steps. First, descriptive statistics relied on medians and interquartile range (IQR) for continuous variables and on frequencies and percentages (%) for categorical variables. Differences in medians were assessed with the Wilcoxon test, while differences in frequencies were evaluated with the Chi-square test. Post hoc analyses were performed when appropriate and p values were adjusted according to false discovery rate in multiple testing.
Second, we used univariable and multivariable logistic regression models to investigate predictors of eGFR < 60 ml/min at 6 and 12 months and predictors of POD-1 AKI development, according to the data available in the literature [3]. Covariate selection for multivariable logistic regression models was performed with a stepwise selection of covariates in both directions (forward and backward). When appropriate, all the models were forced to include the POD-1 AKI as a covariate. In the final multivariable logistic regression model, only variables that ensure a model performance improvement, as indicated by the Akaike Information Criterion (AIC), were included. Ridgeline plots were used to graphically depict smoothed event of interest probabilities in patients with or without AIC. Third, differences between timepoints were considered as follows: Δ1 = POD-1 eGFR – baseline eGFR; Δ2 = 6 months eGFR – POD-1 eGFR; Δ3 = 12 months eGFR – 6 months eGFR. Linear mixed models, considering the non-independent nature of data and the between and within patients’ variation, were used to test the association between main clinical baseline covariates and renal function variation. The interaction between all covariates and time of observation was tested. A multivariable linear mixed model was built. Covariate selection was performed with a stepwise selection of covariates based on AIC. Covariates with a statistically significant association with eGFR variation or interaction with time of observation were considered for stepwise selection. Multivariable models were built within the overall population and after stratification according to AKI. All statistical analyses were performed using R Statistical Software(version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria). All P values were two-tailed, and a p < 0.05 was considered indicative of a statistically significant association.
Results
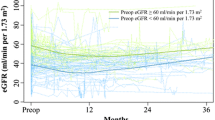
After exclusion criteria’ application, 576 patients were included. Among these, 409 (71.0%) were male, 267 (57.5%) had smoking exposure, 224 (46.4%) had ipsilateral hydronephrosis, 269 (50.8%) harbored muscle invasive (MI)-UTUC and 24(4.6%) exhibited PSM. Median preoperative, POD-1, 6 and 12 months eGFR were 62.2 (IQR 48.1–79.9), 48.2 (IQR 37.6–59.0), 49.8 (IQR 37.8–62.2) and 48.8 (IQR 37.8–64.3) ml/min, respectively. At a median follow-up of 27 months (IQR 15–48), median eGFR was 49.5 ml/min (IQR 37.1–65.2). Overall, 239 (41.5%) patients developed POD-1 AKI, 409 (71.0%) had an eGFR < 60 ml/min at 6 months, and 403 (70.0%) had an eGFR < 60 ml/min at 12 months (Table 1).
In multivariable logistic regression model, age (Odds Ratio, OR 1.05, 95% CI 1.03–1.08, p < 0.001), male gender (OR 0.44, 95% CI 0.25–0.75, p = 0.003), POD-1 AKI(OR 2.88, 95% CI 1.78–4.73, p < 0.001) and preoperative eGFR < 60 ml/min (OR 7.58, 95% CI 4.46–13.29, p < 0.001) were independent predictors of 6 months renal function decline(Table 2). Similarly, preoperative eGFR < 60 ml/min (OR 7.80, 95% CI 4.47–14.12, p < 0.001), age (OR 1.06, 95% CI 1.03–1.09, p < 0.001), CAD (OR 2.68, 95% CI 1.34–5.61, p = 0.007), POD-1 AKI (OR 1.83, 95% CI 1.10–3.08, p = 0.02) were independent predictors of 12 months renal function decline (Table 2). Postoperative chemotherapy did not show a statistically significant effect on eGFR < 60 ml/min at 6 and 12 months (OR 0.87 95% CI 0.50–1.57, p = 0.636, and OR 0.64 95% CI 0.37–1.13, p = 0.116, respectively; data not reported).
In multivariable logistic regression model addressing POD-1 AKI, age (OR 1.04, 95% CI 1.02–1.07, p < 0.001), (absence of-) preoperative hydronephrosis (OR 0.61, 95% CI 0.39–0.93, p = 0.022), preoperative eGFR (OR 1.04, 95% CI 1.03–1.05, p < 0.001), CAD (OR 1.89, 95%CI 1.07–3.36, p = 0.029), and non-MI-UTUC (OR 1.74, 95% CI 1.14–2.67, p = 0.010) were independent predictors of POD-1 AKI development (Supplementary Table 1).
Ridgeline plots illustrating eGFR < 60 ml/min probabilities distribution showed a detrimental effect of POD-1 AKI on eGFR still at 6 and 12 months. Moreover, ridgeline plots highlighted that the effect of POD-1 AKI on eGFR < 60 ml/min is more pronounced at 12 than 6 months (Fig. 1).
Ridgeline plots show the distribution of 6 and 12 month eGFR < 60 ml/min probability in those with or without AKI. Probabilities were estimated from multivariable logistic regression models. Covariates within the models were selected with stepwise regression according to the best AUC. Figures show that density curves picks are more skewed to the right (higher probabilities) for those who experienced AKI
At the previously defined timepoints renal function variation was Δ1 = − 10.2 ml/min (− 25.9; − 2.2), Δ2 = 1.37 ml/min(− 6.2; 9.0), Δ3 = 0.9 ml/min (− 3.6; 5.2 )(Supplementary Fig. 1). Preoperative covariates associated with eGFR variation during follow-up were age (p = 0.019) and hydronephrosis (p < 0.001). Postoperative covariates associated with eGFR variation during follow-up were POD-1 AKI (p < 0.001), and pT-stage (p = 0.024) (Table 3, Supplementary Fig. 2). In the entire cohort, ECOG performance status ≤ 1 was associated with a higher renal function reduction during the time-period (ß − 2.5 ± 1, p < 0.024), and the presence of hydronephrosis was associated with a higher renal function recovery during the time-period (ß 9.2 ± 2.2, p < 0.001). Among patients with POD-1 AKI, the presence of hydronephrosis (ß 2.6 ± 1.3, p = 0.045), and ASA score > 2 (ß 6.7 ± 3.3, p = 0.045) were associated with a lower renal function reduction during the time-period (Supplementary Table 2).
Discussion
We investigated the predictors of postoperative renal function changes in a large multicenter cohort of patients who underwent RNU for UTUC. We found that age, POD-1 AKI and preoperative eGFR were independent predictors of eGFR < 60 ml/min at 6 months after surgery. Similarly, age, CAD, T-stage, POD-1 AKI, and preoperative eGFR were predictors of an eGFR < 60 ml/min at 12 moths after surgery. Additionally, examining the renal function trend over 12 months, patients with ECOG performance status ≤ 1 had a higher renal function reduction, while the presence of hydronephrosis was associated with lower renal function reduction. Our results are in line with those coming from smaller populations involved in the most recent studies. Faba et al. and Hashimoto et al. in retrospective cohorts of 138 and 110 patients, respectively, found that low preoperative eGFR, age, and the absence of hydronephrosis were predictive factors for impaired postoperative renal function after RNU for UTUC [19, 20]. Song et al. showed that high-BMI, low preoperative eGFR, and low contralateral kidney volume were significantly associated with new-onset CKD in 135 patients who underwent RNU [21]. In 2016, Singla et al. in a cohort of 135RNU patients found that after a median follow-up of 28.6 months, patients without hydronephrosis (53%) experienced a greater decline in eGFR following RNU [22]. These findings suggest that previously established contralateral compensatory kidney hypertrophy due to hydronephrosis of the ipsilateral urinary tract facilitates the compensatory role of the remnant solitary kidney.
Interestingly, in 2018, Lee et al. investigated predictors of renal function recovery in a cohort of 118 RNU patients. Half of those with preoperative eGFR < 60 ml/min achieved eGFR recovery within the first 3 years after RNU, and homolateral hydronephrosis was a significant predictor of renal function recovery [23]. The authors commented that prior to definitive surgical intervention, contralateral kidney compensation has begun, and it has facilitated this renal functional recovery along the time [23].
Considering performance status, it has been already demonstrated that ECOG-PS > 1 is associated with worse oncological outcomes and high-grade complications’ rate after RNU for UTUC [24, 25]. We found ECOG > 1 was associated with lower renal function recovery after surgery. This may reflect a general deficiency status including a lower nephrons’ reserve [26], as confirmed in our population where patients with ECOG-PS > 1 had lower preoperative eGFR compared with ECOG-PS ≤ 1 population [ 50.86 (IQR 39.12–64.50) vs. 63.93 86 (IQR 51.79–80.76) ml/min, (p < 0.001); data not shown].
In our study, postoperative chemotherapy did not show a statistically significant effect on eGFR at 6 and 12 months. However, the exact timing of postoperative chemotherapy (immediately after surgery or at recurrence or for disease persistence) was not available for most of the patients. Therefore, we could not include this covariate in multivariable analyses, and its effect on renal function should be elucidated in future studies.
We identified a specific role for POD-1 AKI in renal function decline after RNU. We found that 41.5% of patients experienced AKI on the first POD. In the multivariable model, POD-1 AKI was predicted by absence of hydronephrosis, non-muscle invasive disease and higher preoperative eGFR. In those patients, RNU has a great impact on the residual kidney function, and AKI contributed to a higher probability of developing a 6 and 12 months eGFR reduction. Considering the eGFR trend in AKI population, the presence of hydronephrosis, ASA score > 2 was associated with lower renal function reduction during the time (12 months). Although POD-1 AKI was previously largely investigated after surgery for renal cancer [27, 28], its role was un-investigated after RNU surgery in large-cohort studies. A recent small retrospective single-cohort study investigated how POD-1 AKI influences eGFR reduction, and firstly showed that it was a strong predictor of renal function decline in patients who underwent RNU for UTUC [29]. The authors found that POD-1 AKI affected short- and middle-term renal function impairment, indicating that every effort should be made to prevent POD-1 AKI.
Our findings may have important clinical implications. After RNU for UTUC, patients may require adjuvant chemotherapy for advanced stage or disease progression. Only 50% of patients are still eligible for platinum-based protocols, due postoperative renal function failure [8,9,10] which effects oncological outcomes as recently demonstrated [5]. Early identification of patients at high risk of eGFR reduction after extirpative surgery, for whom adjuvant therapy is no longer feasible, may be addressed by neoadjuvant regimens, resulting in an increase in survival [11]. Conversely, ineligible patients for neoadjuvant therapy have an increased risk of developing renal function decline after RNU and should be treated with kidney-sparing surgery, reducing the morbidity associated with radical surgery without compromising oncological outcomes [30].
The present investigation is retrospective and suffers of its inherent bias. Specifically, the initial cohort coming from 17 tertiary centers was strongly reduced after inclusion of patients with all available interest data only (from1979 to 576 patients).
Our results are innovative and coming from one of the largest populations investigated. We showed that UTUC patients having a nephron reserve before surgery are at high risk to develop a renal function variation after RNU. We also found that POD-1 AKI is a strong predictor of renal function decline. Therefore, a dedicated perioperative management with avoidance of potentially nephrotoxic agents, close monitoring of serum creatinine and urine output should be adopted. Anesthesiologist may contribute to renal damage prevention by reducing the reduction in renal blood and renal hypoxia and preventing hypotension during surgery [31]. When UTUC patients are counseled before treatment, the risk of renal function decline should be extensively explained.
Conclusion
Age, preoperative eGFR and POD-1 AKI are independent predictors of renal function decline at 6 and 12 months after RNU for UTUC. Identifying patients at high risk of renal function decline after RNU allows to provide a correct perioperative patients’ management.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer atatistics, 2021. CA Cancer J Clin 71(1):7–33
Soria F, Shariat SF, Lerner SP et al (2017) Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol 35(3):379–387
Rouprêt M, Babjuk M, Burger M et al (2021) European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol 79(1):62–79
Colin P, Koenig P, Ouzzane A et al (2009) Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int 104(10):1436–1440
Yamada Y, Nakagawa T, Miyakawa J et al (2021) Smaller decline of renal function after nephroureterectomy predicts poorer prognosis of upper tract urothelial carcinoma: a multicentre retrospective study. Jpn J Clin Oncol 51(10):1577–1586
Antonelli A, Minervini A, Sandri M et al (2018) Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer-specific mortality in renal cell carcinoma. Eur Urol 74(5):661–667
Bamias A, Moulopoulos LA, Koutras A et al (2006) The combination of gemcitabine and carboplatin as first-line treatment in patients with advanced urothelial carcinoma: a phase II study of the Hellenic Cooperative Oncology Group. Cancer 106(2):297–303
Birtle A, Johnson M, Chester J et al (2020) Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. The Lancet 395(10232):1268–1277
Shao I-H, Lin Y-H, Hou C-P et al (2014) Risk factors associated with ineligibility of adjuvant cisplatin-based chemotherapy after nephroureterectomy. Drug Des Dev Ther 8:1985
Galsky MD, Hahn NM, Rosenberg J et al (2011) Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 29(17):2432–2438
Leow JJ, Chong YL, Chang SL et al (2021) Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol 79(5):635–654
Tafuri A, Smith DD, Cacciamani GE et al (2020) Programmed death 1 and programmed death ligand 1 inhibitors in advanced and recurrent urothelial carcinoma: meta-analysis of single-agent studies. Clin Genitourin Cancer 18(5):351-360.e3
Meyer JP, Delves GH, Sullivan ME et al (2006) The effect of nephroureterectomy on glomerular filtration rate. BJU Int 98(4):845–848
Kaag MG, O’Malley RL, O’Malley P et al (2010) Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 58(4):581–587
Kaag M, Trost L, Thompson RH et al (2014) Preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma. BJU Int 114(5):674–679
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Bellomo R, Ronco C, Kellum JA et al (2004) Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8(4):1–9
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2011) TNM classification of malignant tumours. John Wiley & Sons
Rodríguez Faba O, Palou J, Breda A et al (2014) Predictive factors for impaired renal function following nephroureterectomy in upper urinary tract urothelial cell carcinoma. Urol Int 92(2):169–173
Hashimoto T, Ohno Y, Nakashima J et al (2015) Prediction of renal function after nephroureterectomy in patients with upper tract urothelial carcinoma. Jpn J Clin Oncol 45(11):1064–1068
Song W, Sung HH, Han DH et al (2017) The effect of contralateral kidney volume on renal function after radical nephroureterectomy: Implications for eligibility for neoadjuvant chemotherapy for upper tract urothelial cancer. Urol Oncol 35(3):114.e1-114.e7
Singla N, Hutchinson R, Haddad A et al (2016) Preoperative hydronephrosis is associated with less decline in renal function after radical nephroureterectomy for upper tract urothelial carcinoma. Can J Urol 23(4):8334–8341
Lee BH, Zabor EC, Tennenbaum D et al (2018) Renal function recovery after radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 36(2):257–263
Eismann L, Bohn L, Buchner A et al (2022) Age and ECOG performance status as predictors of survival of patients with upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy. Urol Int. https://doi.org/10.1159/000522295
Kocher NJ, Canes D, Bensalah K et al (2020) Incidence and preoperative predictors for major complications following radical nephroureterectomy. Transl Androl Urol 9(4):1786–1793
Otobe Y, Rhee CM, Nguyen M et al (2022) Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr Opin Nephrol Hypertens 31(1):109–128
Martini A, Cumarasamy S, Beksac AT et al (2018) A nomogram to predict significant estimated glomerular filtration rate reduction after robotic partial nephrectomy. Eur Urol 74(6):833–839
Antonelli A, Mari A, Tafuri A et al (2022) Prediction of significant renal function decline after open, laparoscopic, and robotic partial nephrectomy: external validation of the Martini's nomogram on the RECORD2 project cohort. Int J Urol
Tafuri A, Odorizzi K, Di Filippo G et al (2021) Acute kidney injury strongly influences renal function after radical nephroureterectomy for upper tract urothelial carcinoma: a single-centre experience. Arch Ital Urol Androl 93(1):9–14
Seisen T, Peyronnet B, Dominguez-Escrig JL et al (2016) Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol 70(6):1052–1068
Zarbock A, Koyner JL, Hoste EAJ et al (2018) Update on perioperative acute kidney injury. Anesth Analg 127(5):1236–1245
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
AT, M Marchioni, M Moschini, MF, AA: contributed to protocol/project development. CC, K O, RT, AV, DA, UC, AP, FT, MC, LMA, LB, GN, EL, FM, F Crocerossa, F Cantiello, DD, FDC, FDG: contributed to data collection or management. M Marchioni, AT: contributed to data analysis. AT, M Marchioni, CC, AS, AA: contributed to manuscript writing/editing. AM, FDM, RS, PP, SC, ODC, PB, FP, PD, EM, FS, PG, GL, CT, GM, MB, CT, AS, AG, M Moschini, SFS, MAC, MDN, AM, MF, LS, MAC,VP, AA: contributed to other: critical manuscript revision. AA, VP: contributed to other: supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
eGFR variations at the defined timepoints: baseline, postoperative day 1, 6 months, and 12 months. Differences between each timepoint and the previous one were tested with the Wilcoxon test after adjusting for multiple hypothesis testing according to the false discovery rate method (PDF 217 KB)
Supplementary Fig. 2
eGFR variation according to clinically meaningful covariates. The eGFR variation was calculated as the difference between each timepoint and the previous timepoint (∆1 = eGFR POD I – eGFR preoperative; ∆2 = eGFR at 6 month – eGFR POD III, ∆3 = eGFR 12 month – eGFR 6 month). Differences between each group were evaluated with the Wilcoxon test after adjusting for multiple hypothesis testing according to the false discovery rate method (PDF 844 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tafuri, A., Marchioni, M., Cerrato, C. et al. Changes in renal function after nephroureterectomy for upper urinary tract carcinoma: analysis of a large multicenter cohort (Radical Nephroureterectomy Outcomes (RaNeO) Research Consortium). World J Urol 40, 2771–2779 (2022). https://doi.org/10.1007/s00345-022-04156-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04156-3