Abstract
Exercise training can induce adaptive changes to tendon tissue both structurally and mechanically; however, the underlying compositional changes that contribute to these alterations remain uncertain in humans, particularly in the context of the ageing tendon. The aims of the present study were to determine the molecular changes with ageing in patellar tendons in humans, as well as the responses to exercise and exercise type (eccentric (ECC) and concentric (CON)) in young and old patellar tendon. Healthy younger males (age 23.5 ± 6.1 years; n = 27) and older males (age 68.5 ± 1.9 years; n = 27) undertook 8 weeks of CON or ECC training (3 times per week; at 60% of 1 repetition maximum (1RM)) or no training. Subjects consumed D2O throughout the protocol and tendon biopsies were collected after 4 and 8 weeks for measurement of fractional synthetic rates (FSR) of tendon protein synthesis and gene expression. There were increases in tendon protein synthesis following 4 weeks of CON and ECC training (P < 0.01; main effect by ANOVA), with no differences observed between young and old males, or training type. At the transcriptional level however, ECC in young adults generally induced greater responses of collagen and extracellular matrix-related genes than CON, while older individuals had reduced gene expression responses to training. Different training types did not appear to induce differential tendon responses in terms of protein synthesis, and while tendons from older adults exhibited different transcriptional responses to younger individuals, protein turnover changes with training were similar for both age groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing is associated with progressive structural and mechanical changes to collagen-rich tissues such as tendon [1], and in elderly individuals, this can have negative impacts on tissue function and contribute to functional disabilities and injuries [2,3,4]. Tendon tissues are predominantly composed of collagen and non-collagenous extracellular matrix (ECM) components including proteoglycans, the turnover of which is regulated by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). Resistance exercise (RE) appears to attenuate age-related functional declines in tendons by altering tendon mechanical properties [2]; however, the molecular changes that underlie these responses are uncertain.
The majority of data regarding the effects of ageing on tendon structural and functional properties have been generated from in vitro and animal models. Tenocytes cultured from mice of different ages revealed a decrease in rates of proliferation with age and a reduction in expression of stem cell markers [5, 6]. In rat tendons, Kostrominova et al. [7] observed decreased gene expression of collagen types I, III and V, as well as key proteoglycans and ECM-related proteins, with increasing age. At the structural level, ageing patellar tendons from mice were demonstrated to have deterioration of viscoelastic properties, with reduced collagen fibre alignment and altered tenocyte shape [8]. In humans, transcriptomic analysis of Achilles tendons with ageing found distinct differences between old and young tendons, with altered expression of genes important in cell growth and cell cycle pathways [9].
While it is known that RE can result in tendon tissue adaptive changes both structurally and mechanically [10], the underlying regulatory factors that contribute to these changes remain uncertain, particularly in the context of the ageing tendon. In humans, acute (4 h) RE resulted in reduced expression of collagen type I and III and MMPs [11], while additional studies in human tendon have shown that acute exercise induced few changes in gene expression (related to collagen, growth factors and matrix proteins) compared to the changes in muscle [12]. In relation to ageing, a recent study in older rats demonstrated that resistance training increased growth factor expression and proteoglycan content in tendons of old rats [13], while 10 weeks of uphill running resulted in reduced tendon stiffness and increased gene expression of collagen turnover related genes in Achilles tendons of older mice [14].
Studies have also focused on the effects that different exercise intensities and types may have on tendon adaptation. In the work of Xu et al. [15], moderate intensity treadmill running induced collagen synthesis and collagen fibril organisation, while high intensity running appeared to cause collagen degradation and disturbance in rat Achilles tendons. Two further studies compared 4 days of concentric (CON) and eccentric (ECC) training in female rats (as the greater absolute mechanical load in ECC vs. CON could produce differential anatomical, cellular and molecular responses), observing that acute exercise upregulated IGF-1 isoforms in Achilles tendon, as well as genes related to collagen synthesis and processing; however, no difference between contraction mode was observed [16, 17]. In humans, studies have suggested that different contraction types and intensities can differentially influence tendon mechanical properties in young and older adults [18, 19]; however, the cellular/molecular factors underlying those differences require further study.
The aims of the present study were to determine the molecular changes with ageing in tendons in humans (specifically, at the level of tendon protein synthesis and gene expression changes), as well as the subsequent impacts of 4 and 8 weeks of training. ECC and CON training types were compared in order to evaluate whether there were any differences in tendon remodelling based on training mode. Our hypothesis was that older tendons would exhibit differences in molecular properties (including collagen and ECM-related molecular changes), and that training would induce tendon cellular adaptations in both age groups, but that these changes would be attenuated in older individuals.
Methods
Ethical approval, participants, study protocol and sample collection
Fifty-four healthy, recreationally active individuals were recruited, 27 of which were younger males (age (mean ± SD): 23.5 ± 6.1 years; body weight: 74.4 ± 13.2 kg) and 27 older males (age: 68.5 ± 1.9 years; body weight: 78.7 ± 9.4 kg). All participants underwent a full medical screening prior to enrolment, whereby those with any musculoskeletal, metabolic, respiratory, neurological or cardiovascular medical conditions were excluded from the study. None of the participants utilised in this study was deemed as frail or having had a history of falls. All participants provided written informed consent to this study. The study was approved by the University of Nottingham Ethics Committee and was performed in accordance with the Declaration of Helsinki (approval number B13032014 SoMSGEM).
For both younger and older males, participants were randomised into 3 groups: eccentric (ECC; n = 9), concentric (CON; n = 9) or control (n = 9). As tendon biopsy limitations meant pre-training samples could not be collected from each individual, inclusion of the control (non-trained) group provided baseline measures and basal enrichment measurements. CON and ECC groups underwent 8 weeks training with 3 sessions per week, utilising a custom leg press machine, which enabled the isolation of CON and ECC only contractions (a set-up that has previously been described in detail [20]). The first 4 weeks was bilateral and was switched to unilateral for final 4 weeks, due to the tendon biopsy acquired. Each exercise bout consisted of 4 sets of 12–15 repetitions at 60% load of either CON or ECC 1 repetition maximum (1RM), with 2-min rest between sets. Training load was monitored and progressively increased according to changes in ECC or CON 1RM. Functional outcomes of the training responses, and tendon biomechanical properties for this study, have previously been described in Quinlan et al. [21]. Participants were provided with D2O which was taken as a bolus of < 250 ml (based on body weight) followed by < 100 ml per week. Two saliva samples were collected each week for determination of body water enrichment. At the 4- and 8-week timepoints, patellar tendon biopsies were collected from the participants (60 min after a training session). Briefly, under sterile conditions and ultrasound guided, an automatic disposable 14 G Bard Monopty Biopsy Instrument (Bard Inc., Covington, GA, USA) was utilised to obtain ~ 10 mg of tissue from the proximal patellar tendon via a single pass (Supporting Fig. 1). The tissue was consequently snap frozen in an RNase-free tube and placed in a − 80 °C freezer for future analysis.
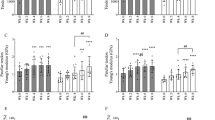
Expression of extracellular matrix (ECM)-related and regulatory growth factor genes between young and old individuals in tendon tissue. Expression of genes related to collagen proteins (A), ECM remodelling (B), regulatory growth factors (C), and structural ECM-related proteins and proteoglycans (D) were compared between old and young untrained individuals in tendon tissue, with data normalised to actin expression and presented as fold change versus young group (n = 9 per group). Results are displayed as mean + SEM. aP < 0.05 versus young group
RNA extraction, cDNA synthesis and real-time PCR
Portions of tendon samples (weights ~ 4–8 mg) were placed in 2-ml tubes along with 500 µl ice-cold TRI Reagent® (Sigma-Aldrich, UK) and 5-mm stainless steel beads (Qiagen, UK). Tissue was lysed by homogenising for 2–3 rounds of 30 s using a TissueLyser II (Qiagen, UK), until tissue was visibly homogenised. Samples were kept on ice in-between each round of homogenisation.
Following homogenisation, RNA extraction was carried out according to the TRI Reagent® manufacturer’s protocol (Sigma-Aldrich, UK). The RNA pellet was resuspended in 10 µl RNase-free water, and RNA quantity and quality (260:280 nm and 260:230 nm ratios) were determined using a NanoDrop™ 2000 (Thermo Fisher Scientific). At the stage of phase separation, the lower (protein-containing) phase was kept for protein isolation to be used for the stable isotope enrichment analyses (see below).
RNA (500 ng) was reverse transcribed using the high-capacity cDNA synthesis kit (Applied Biosystems, Thermo Fisher Scientific), which was diluted 1:5 using RNase-free water. For targeted real-time PCR measurements, 1 µl of cDNA was loaded into 384-well plates (in duplicate) with SYBR™ Select Master Mix (Applied Biosystems, Thermo Fisher Scientific) and gene-specific primers (for list of primers used, see Table 1). Genes were selected to represent a range of biological pathways related to structural and regulatory proteins in tendon. The ΔΔCt method [22] was used to quantify mRNA expression, with ACTB expression being used for normalisation (during initial tests, a total of 5 potential housekeeping genes were assessed, including PPIA, ACTB, RPL13A, TBP and UBC, and ACTB was the most stable gene across age and training type).
Protein extraction, hydrolysis and determination of protein-bound alanine enrichment
Due to the limited size of tendon biopsies, we aimed to measure protein-bound alanine enrichment from the protein fraction that was generated during the TRI Reagent® RNA extractions. Initial tests determined that comparable fractional synthetic rates (FSR) were generated using this method and protein extraction methods that have previously been published ([23]; data not shown). In addition, initial tests aimed to fractionate the samples into alkali-soluble and alkali-insoluble parts; however, tests with human tendons did not yield consistent results/sufficient alkali soluble protein for mass spectrometric analysis. Protein was extracted from the lower phase of TRI Reagent® extractions by adding isopropanol and centrifuging for 10 min at 12,000 × g. Protein-containing pellets were washed twice with ethanol, and then protein was hydrolysed by incubating in 0.1 mol L−1 HCl in Dowex H+ resin overnight at 110 °C. Samples were isolated by passing through Dowex resin columns, eluted with 2 mol L−1 NH4OH and dried down. Amino acids were derivatised as their N-methoxycarbonyl methyl esters. Protein-bound alanine enrichment was determined by gas chromatography-pyrolysis-isotope ratio mass spectrometry (Delta V Advantage; Thermo Scientific). Within the same sample run, the samples were used to determine the ratio of hydroxyproline/proline.
Protein synthesis was calculated from the incorporation of deuterium-labelled alanine into protein, with enrichment of body water as precursor labelling, as previously described [24]. Fractional synthetic rate (FSR) was calculated using the following equation:
where t is time in days, APEAla is the deuterium enrichment of protein-bound alanine and APEP is the average enrichment of the body water precursor, i.e. saliva, over the time between biopsies. Since there were no biopsies collected at time = 0, additional tendon biopsies were collected from individuals who did not receive D2O, and these were used for baseline enrichment.
Body water enrichment
Enrichment of body water was determined using 50 µl saliva which was evaporated onto inverted autosampler vials (4 h at 100 °C) and then placed upright onto ice. Enrichment of body water was determined by injecting into a high-temperature conversion elemental analyser (Thermo Finnigan, Thermo Scientific, Hemel Hempstead, UK) connected to a Delta V Advantage Isotope Ratio Mass Spectrometer (Thermo Finnigan, Thermo Scientific).
Tendon cross sectional area (CSA)
Transversal magnetic resonance imaging (MRI) scans were performed at each timepoint for all training groups via a 3 T scanner. Participants were lying supine, with the knee joint fixed at 180°. Slice thickness was set at 3 mm with no interslice gap. Tendon CSA values were calculated to correspond to the biopsy site (Supporting Fig. 1). A total of three CSA images were manually measured via offline digital analysis software (OsiriX Lite 9.5.2, Pixmeo SARL), and an averaged value was generated for further analysis. Note that one elderly ECC was unable to have an MRI scan due to medical reasons and hence n = 8 for the old ECC group, whereas n = 9 for all others.
Statistical analyses
For gene expression analyses, genes were sub-categorised based on ontology, and 1-way ANOVA was used to assess main effects of each training type for each age group and timepoint. Sidak’s multiple comparison tests were used to determine any individual changes in gene expression between groups (P < 0.05 was considered statistically significant). For protein synthesis analyses, exercise and age comparisons were made using two-way ANOVA with Sidak’s multiple comparisons tests. Statistical tests were performed using Prism version 7 (GraphPad Software Inc., San Diego, CA, USA).
Results
Gene expression comparisons in tendon tissue between young and older males
An age comparison of genes related to tendon structure, remodelling and turnover was evaluated in untrained young and old individuals. Individually, there were no significant differences between young and older males for collagen encoding genes; however, there was an overall (main effect by ANOVA) downregulation of collagen genes with age (P < 0.05 vs. young), and there was a significant decrease in LOX expression in older males (0.5 ± 0.1-fold; P < 0.05 vs. young; Fig. 1A). There were no differences between young and older males for genes involved in ECM remodelling or regulatory growth factors (Fig. 1B, C, respectively). In terms of tendon structural-related genes, there were specific decreases in DCN (0.6 ± 0.1-fold; P < 0.05 vs. young), ECM1 (0.5 ± 0.1-fold; P < 0.05 vs. young) and VCAN (0.5 ± 0.1-fold; P < 0.05 vs. young) mRNA expression in older individuals (Fig. 1D).
Protein synthesis and collagen proline hydroxylation in tendon tissue following 4- and 8-week eccentric or concentric training in young and older males
There were no differences in tendon protein synthesis between young and older males in the control group (Fig. 2A). Following 4 weeks of CON training, there was a main effect (P < 0.01 by ANOVA) of training (increase) on tendon protein synthesis rates for both the young and old groups (0.23 ± 0.09%/day vs. 0.084 ± 0.012%/day in young CON vs. young controls; 0.25 ± 0.06%/day vs. 0.085 ± 0.007%/day in old CON vs. old controls; Fig. 2A), whereas by 8 weeks of CON training, protein synthesis rates of tendon were no different from the control group, for both young and old adults (Fig. 2B). Following 4 weeks of ECC training, there was a main effect (P < 0.01 by ANOVA) of exercise (increase) on tendon protein synthesis rates for both the young and old groups (Fig. 2A), and post hoc testing showed a significant increase in FSR in the old group (0.13 ± 0.014%/day vs. 0.085 ± 0.007%/day; P < 0.05). By 8 weeks of ECC training, protein synthesis rates of tendon were no different from the control group, for both young and old adults (Fig. 2B).
Fractional synthetic rates (FSR) of protein synthesis in young and old individuals following 4- and 8-week eccentric or concentric training in tendon tissue. Tendon protein synthesis rates with 4- (A) and 8- (B) week concentric training or eccentric training in young and older males, compared to control group. n = 9 per group. aP < 0.05 versus control group. Tendon protein 2H incorporation at 4 and 8 weeks in young and old combined (C). Baseline (black bar) represents 2H levels in tendon prior to D2O consumption
The ratio of hydroxyproline to proline in tendon tissue was assessed with training and across age groups. There were no significant effects of either training type at 4 or 8 weeks (Fig. 3); however, there was a main effect of an increase in hydroxylated proline with increased age (P < 0.01 by ANOVA; Fig. 3).
Hydroxyproline to proline ratios in young and old individuals following 4- and 8-week eccentric or concentric training in tendon tissue. Ratio of hydroxyproline to proline in tendon tissue with 4- (A) and 8- (B) week concentric training or eccentric training in young and older males, compared to control group. n = 9 per group
Gene expression in tendon tissue following 4- and 8-week eccentric or concentric training in young males
Genes encoding collagen proteins and factors that regulate collagen cross-linking were assessed in tendons of young males following 4 weeks ECC or CON training. Following 4 weeks CON training, there were increases in COL1A1 (3.4 ± 0.5-fold; P < 0.001 vs. control) and COL6A1 (2.0 ± 0.4-fold; P < 0.05 vs. control) expression relative to young controls, while other collagen genes measured were unchanged (Fig. 4A). With ECC training, however, increases in mRNA expression of COL1A1, COL6A2, COL6A3, COL12A1, COL14A1, LOX and P4HA1 were observed, relative to untrained individuals (Fig. 4A). COL1A1, COL6A2, COL6A3, COL12A1 and LOX were also all upregulated with ECC relative to the CON group. Following 8 weeks of training, there were no changes in gene expression in the CON group, and in contrast to the changes observed after 4 weeks of training, the majority of genes were unchanged with ECC training, with only an increase in COL1A1 expression observed (2.4 ± 0.4-fold; P < 0.05 vs. control; Supporting Fig. 2).
Expression of extracellular matrix (ECM)-related and regulatory growth factor genes in young individuals following 4-week eccentric (ECC) or concentric (CON) training in tendon tissue. Expression of genes related to collagen proteins (A), ECM remodelling (B), regulatory growth factors (C) and structural ECM-related proteins and proteoglycans (D) were compared in young individuals following 4-week ECC or CON training in tendon tissue, with data normalised to actin expression and presented as fold change versus controls (n = 9 per group). Results are displayed as mean + SEM. aP < 0.05 versus control group. bP < 0.05 versus CON group
Gene expression of selected TIMPs and MMPs (encoding key ECM remodelling proteins) were also measured with training in patellar tendon tissue of young individuals. Expression of TIMP2 mRNA was increased with CON training (1.3 ± 0.1-fold; P < 0.05 vs. control), while TIMP1, TIMP3 and TIMP4, and all MMPs measured, were unaltered (Fig. 4B). With ECC however, TIMP1, TIMP2 and TIMP4 mRNA were all elevated compared to control, while increases in MMP2 and MMP3 gene expression were also observed (Fig. 4B). Expression of ADAMTS1, however, was increased with CON training (1.6 ± 0.2-fold; P < 0.05 vs. control), with no change observed in the ECC group.
Regulatory growth factor genes were also assessed in tendons of young individuals following 4-week CON or ECC training. None of the measured growth factor genes was altered with CON training; however, IGF1 and TGFB1 were significantly increased with ECC training (2.0 ± 0.5-fold; P < 0.05 vs. control and 1.6 ± 0.2-fold; P < 0.01 vs. control, respectively; Fig. 4C). There were, however, no changes in CTGF, VEGF, PDGF or FGF2 in tendons of trained individuals relative to controls (Fig. 4C).
Genes encoding structural ECM-related proteins and proteoglycans were measured, with no observed changes seen with 4-week CON training. With ECC however, increases in DCN (3.0 ± 0.4-fold; P < 0.001 vs. Control), FN1 (2.1 ± 0.4-fold; P < 0.05 vs. Control) and VCAN (3.9 ± 0.6-fold; P < 0.001 vs. Control) were observed in tendon relative to untrained controls (Fig. 4D).
Gene expression in tendon tissue following 4- and 8-week eccentric or concentric training in older males
Gene expression changes were assessed in tendons of older males following 4 weeks ECC or CON training. There were no changes in genes encoding collagen proteins for either training type at 4 weeks (Fig. 5A). Similarly, genes encoding ECM remodelling proteins and regulatory growth factors were unaltered with either training type in older males after 4 weeks (Fig. 5B, C).
Expression of extracellular matrix (ECM)-related and regulatory growth factor genes in older individuals following 4-week eccentric (ECC) or concentric (CON) training in tendon tissue. Expression of genes related to collagen proteins (A), ECM remodelling (B), regulatory growth factors (C) and structural ECM-related proteins and proteoglycans (D) were compared in older individuals following 4-week ECC or CON training in tendon tissue, with data normalized to actin expression and presented as fold change versus controls (n = 9 per group). Results are displayed as mean + SEM. aP < 0.05 versus control group. bP < 0.05 versus CON group
In terms of genes encoding structural proteins and proteoglycans, 4 weeks of CON training resulted in an increase in FN1 (2.8 ± 0.8-fold; P < 0.05 vs. control) and VCAN (3.1 ± 1.1-fold; P < 0.05 vs. control) expression, while TNC expression was decreased (0.6 ± 0.1-fold; P < 0.05 vs. control), and other measured genes remained unchanged (Fig. 5D). With ECC training, no genes encoding structural-related ECM proteins were altered after 4 weeks.
At 8 weeks of training, there were no changes in gene expression for most targets analysed, although there was a decrease in THBS2 and ECM1 mRNA with CON training and a significant decrease in THBS1 expression (0.5 ± 0.1-fold; P < 0.05 vs. control) following 8-week ECC training (Supporting Fig. 3).
Proximal CSA changes in response to 4 and 8 weeks of eccentric or concentric training
Proximal tendon CSA was significantly increased following 8 weeks of CON (0.71 ± 0.04cm2 vs. 0.75 ± 0.05cm2, P < 0.01) and ECC training in the young groups (0.75 ± 0.09cm2 vs. 0.78 ± 0.08cm2, P < 0.05) (Fig. 6). However, no changes were observed in either CON or ECC elderly groups.
Proximal tendon cross-sectional area (CSA) in both young and older individuals following 4- and 8-week eccentric (ECC) or concentric (CON) training. The tendon proximal CSA in young (A) and old (B) individuals following ECC or CON training (n = 9 for all except Old ECC, n = 8). a = P < 0.05 vs. baseline, b = P < 0.01 vs. baseline
Discussion
Although it is known that exercise training can result in adaptive changes to tendon tissue at the structural, functional [25] and metabolic level [26], the underlying regulatory factors that contribute to these alterations are poorly understood in humans, particularly in the ageing tendon. In the present study, we aimed to examine the molecular changes with ageing in patellar tendons in humans, as well as the impact of 4- and 8-week eccentric (ECC) and concentric (CON) training. We found that while ECC appeared to induce a greater remodelling response of tendon than CON at the level of gene expression, this did not impact on tendon protein turnover, since increases in protein synthesis were comparable between training types in young and older individuals. Furthermore, despite transcriptional changes indicating reduced responsiveness of training in older adults, protein synthesis responses with training were similar for both age groups. These findings provide novel insights into tendon adaptation to mechanical loading at the molecular level with human ageing.
Age differences in tendon protein metabolism and gene expression
In the present study, we sought to determine basal age-related differences in tendon properties in terms of protein metabolism and gene expression. In the group of non-exercised individuals, there was no difference in protein turnover throughout the period studied. In terms of mRNA changes, there was an overall downregulation of tendon collagen genes, and specific proteoglycan genes (DCN, ECM1 and VCAN) in older age, which is in general agreement to previous work in rat tendons with ageing [7]. While these changes did not appear to impact on global protein turnover, they could feasibly contribute to specific structural and/or mechanical changes in tendons of older individuals.
One observed age-related difference was the increase in proline hydroxylation observed in tendons of older males (but no alterations with training), an observation that has previously been reported [23]. Proline hydroxylation is an important component of collagen formation since it provides structural stability of the triple helix [27, 28]. Proline hydroxylation may also have additional biological functions, since many collagen-interacting proteins contain hydroxyproline functional motifs [28]. Thus, the potential impacts of increased proline hydroxylation in tendon collagen, and how this may have mechanical/functional consequences, require further study. We recently observed, in these same individuals [20], that while there were adaptations in the tendon with training in both the older and younger groups, in terms of changes in tendon biomechanical properties (tendon stiffness and Young’s modulus), the older tendon appeared to take longer to maximally respond. While the underlying factors that contribute to this observation are not clear, it is possible that the age-related differences in collagen-related gene expression and hydroxyproline could (at least partially) influence tendon mechanical properties.
Tendon tissue remodelling following 4- and 8-week eccentric or concentric training in young males
Tendon tissue changes in protein synthesis and gene expression were measured in young males undergoing 4 and 8 weeks of ECC or CON exercise, relative to untrained controls. After 4 weeks of training, tendon protein synthesis was increased in young males, with no significant differences between training types. Tendon collagen synthesis has been previously demonstrated to increase in response to acute exercise in healthy young males [29], and longer-term training [30]; however, the effects of specific contraction types on tendon protein synthesis remain poorly understood. In skeletal muscle, 4-week ECC or CON training did not result in any differences in protein synthesis [31] and not even after 8 weeks of the same training program [32], while a study in rats reported that tendons were not responsive to contraction type in terms of transcriptional changes in collagen and anabolic growth factor genes [16, 17]. Our study provides further insight into the adaptive responses of tendon tissue with training, specifically, that in terms of protein synthesis, tendon may be unresponsive to contraction type in humans, but shown to be responsive to contraction load.
Gene expression changes (related to collagen, growth factor, proteoglycan and ECM remodelling genes) were also evaluated in tendon tissue with ECC and CON training in young individuals, with the aim of further understanding the molecular changes underlying tendon remodelling with exercise. We found that changes with exercise appeared to occur early in tendon (with few changes seen after 8 weeks of training), while ECC appeared to induce greater remodelling responses in tendon than CON (in terms of gene expression). A previous study in humans demonstrated that acute RE induced changes in tendon gene expression of collagen and matrix remodelling genes [11]. In that study, there were no observed changes in proteoglycan genes, although previous work has demonstrated tendon proteoglycans to be responsive to mechanical loading [33, 34]. Several MMP/TIMP genes were also induced after 4 weeks of ECC training in younger adults, including MMP2 and MMP3. MMP proteins are responsible for ECM breakdown, and MMP2 and MMP3 have been previously shown to be upregulated with mechanical stimuli [35, 36]. Whether the different contraction type with ECC exercise induced greater ECM breakdown in the tendon requires further evaluation. Overall, ECC appeared to induce a greater gene expression response than CON training in young individuals, but these differences were not reflected in overall rates of protein synthesis. It should be noted that the gene expression changes reflect a single point in time, while the protein synthesis measures represent cumulative changes over a 4-/8-week period. However, while ECC appeared to have a greater impact on gene expression, changes in tendon CSA showed no difference between CON and ECC training. Both training modalities increased the CSA in the tendon’s proximal region, which corresponded to the biopsied site; this region of the patellar tendon has previously been reported to increase in size in response to mechanical loading [37, 38]. This tendon size increase would at least partly be accounted for by hypertrophy of the tendon, as suggested via the increased expression of COL1A1 and the increase in tendon FSR that occurred in both groups. However, it should also be considered that the patellar tendon’s proximal region in particular is subjected to large compressive loading due to it wrapping around the patellar bone [39, 40]. Compressive loads are linked with the expression of large aggregating proteoglycans such as versican and biglycan, which increase water content in the tendon to provide greater resistance to compression.
Tendon tissue remodelling following 4- and 8-week eccentric or concentric training in older males
While RE has been shown to improve tendon mechanical properties [25] and induce structural changes in humans [2], the specific adaptations with exercise in older individuals, and especially the mechanisms responsible for these changes, remain incompletely understood. In the present study, there were relatively few altered genes following either 4 or 8 weeks ECC or CON training in older males, compared to the young group. However, despite transcriptional changes indicating reduced responsiveness of training in older adults, protein synthesis responses with training were similar for both age groups. As with the ECC versus CON comparisons, there was no relation between gene expression responses to training in the older group, and protein synthesis responses, although temporal differences in gene responses could account for observed differences. Overall, the present work demonstrates that similar adaptive changes, in terms of tendon protein synthesis, were observed in healthy older individuals to younger adults. Similarly, we also observed no change in proximal tendon CSA. This is in contrast to the younger groups, but there are some potential explanations as to why this is the case. It is possible that as the elderly individuals have a higher proximal tendon CSA value at baseline; while an increase in proximal CSA is possible in the young, this may not the case in the elderly groups. Indeed, larger tendon CSA values have previously been reported in elderly compared to younger individuals [41, 42], likely due to prolonged habitual loading.
This study advances our understanding on the mechanisms of tendon collagen remodelling with exercise and age; however, our study is not without some limitations. Due to the complexity and extent of collagen crosslinking, there is evidence suggesting the existence of both dynamic and inactive collagen pools within ECM, resulting in incomplete turnover [43, 44]. This has been demonstrated in the liver and muscle of mice [43, 44], yet complete turnover has been predicted in rodent bone collagen measurements [45]. However, due to the very slow turnover rate of collagen in ECM tissues, these primarily rely on predicted plateaus, and therefore extremely long labelling studies are required to fully determine the size of any inactive collagen pool, especially in humans. That being said, the possible impact of inactive and heterogeneous collagen pools on turnover measurements needs to be acknowledged.
Our data in human patellar tendon represents initial tracer incorporation (~ 5% fraction new) and only follows the very early phase of a rise to plateau curve; we believe in this early phase that it is both valid and appropriate to calculate FSR assuming 100% renewal. Currently, there is no human data that defines the size or relative proportion of inactive and dynamic collagen pools, nor how they might change in response to a chronic intervention. Though we would postulate that any increase in the proportion of the active pool, e.g. in response to loading/exercise, would result in greater overall incorporation of tracer resulting in a greater FSR, a reduction in the active pool would result in less tracer incorporation and an apparent reduction in FSR. Given our intervention is relatively short in relation to the time involved for the tendon collagen pool to fully turnover, we contend that the data reflect the physiological responses of tendon to eccentric and concentric exercise. There is evidence from human Achilles tendon studies that some tendon made during early growth remains throughout life [46]. However, tendon has demonstrated active collagen synthesis at rest, with an increase in the rate of synthesis in response to exercise [29]. That being said, if large proportions of tendon are inactive, then FSR would be increased [43], yet comparisons across conditions would still be warranted.
Conclusions
In summary, we report a general downregulation of tendon collagen/cross-linking genes, and specific proteoglycan genes in older age, while there was evidence of increased tendon proline hydroxylation with age. While it appeared that the older tendon was less responsive to exercise training, in terms of a molecular remodelling perspective, protein synthesis was similar for both age groups. Finally, changes in remodelling with exercise appeared to occur early in tendon (with few changes seen with 8 weeks of training), and although ECC appeared to induce greater remodelling of tendon than CON at the molecular level, there was no observable difference in terms of protein turnover between training types. Nevertheless, similar responses of protein turnover with ECC training relative to CON, in relation to the lower metabolic cost and greater application of strain that can be achieved with this training type, could provide support for an overall more beneficial response of ECC training for tendon tissue. The present investigation provides novel insights into the influences of longer-term training on cellular responses in the human tendon. Future work should aim to investigate whether/how these cellular changes to tendon may/may not translate to functional outcomes with training in older individuals.
References
Quinlan, J.I.; Maganaris, C.N.; Franchi, M. v.; Smith, K.; Atherton, P.J.; Szewczyk, N.J.; Greenhaff, P.L.; Phillips, B.E.; Blackwell, J.I.; Boereboom, C.; et al. Muscle and tendon contributions to reduced rate of torque development in healthy older males. J Gerontol - Series A Biol Sci Med Sci 2018. https://doi.org/10.1093/gerona/glx149.
Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP. Effect of aging and exercise on the tendon. J Appl Physiol. 2016;121:1353–62.
Tuite DJ, Renström PAFH, O’Brien M. The aging tendon. Scand J Med Sci Sports. 2007;7:72–7. https://doi.org/10.1111/j.1600-0838.1997.tb00122.x.
Birch HL, Peffers MJ, Clegg PD. Influence of ageing on tendon homeostasis. Adv Exp Med Biol. 2016;920:247–60.
Tsai WC, Chang HN, Yu TY, Chien CH, Fu LF, Liang FC, Pang JHS. Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of P27. J Orthop Res. 2011;29:1598–603. https://doi.org/10.1002/jor.21418.
Zhang J, Wang JHC (2015) Moderate exercise mitigates the detrimental effects of aging on tendon stem cells. PLoS One 10. https://doi.org/10.1371/journal.pone.0130454.
Kostrominova TY, Brooks Sv. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Omaha). 2013;35:2203–14. https://doi.org/10.1007/s11357-013-9514-2.
Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo Rv, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. https://doi.org/10.1016/j.matbio.2012.11.005.
Peffers, M.J.; Fang, Y.; Cheung, K.; Wei, T.K.J.; Clegg, P.D.; Birch, H.L. Transcriptome analysis of ageing in uninjured human achilles tendon. Arthritis Res Ther 2015, 17. https://doi.org/10.1186/s13075-015-0544-2.
Heinemeier KM, Skovgaard D, Bayer ML, Qvortrup K, Kjaer A, Kjaer M, Magnusson SP, Kongsgaard M. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol. 2012;113:827–36. https://doi.org/10.1152/japplphysiol.00401.2012.
Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Døssing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory MRNA expression. J Appl Physiol. 2009;106:468–75. https://doi.org/10.1152/japplphysiol.91341.2008.
Heinemeier, KM.; Bjerrum, SS.; Schjerling, P.; Kjaer, M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports 2013, 23. https://doi.org/10.1111/j.1600-0838.2011.01414.x.
Marqueti RC, Durigan JLQ, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER, Selistre-De-Araujo HS. Effects of aging and resistance training in rat tendon remodeling. FASEB J. 2018;32:353–68. https://doi.org/10.1096/fj.201700543R.
Wood LK, Brooks Sv. Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J Orthop Res. 2016;34:346–53. https://doi.org/10.1002/jor.22824.
Xu SY, Bin He Y, Deng SY, Liu SY, Xu L, Ni GX. Intensity-dependent effect of treadmill running on rat Achilles tendon. Exp Ther Med. 2018;15(5377):5383. https://doi.org/10.3892/etm.2018.6084.
Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–81. https://doi.org/10.1152/japplphysiol.00866.2006.
Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–16. https://doi.org/10.1113/jphysiol.2007.127639.
Malliaras P, Kamal B, Nowell A, Farley T, Dhamu H, Simpson V, Morrissey D, Langberg H, Maffulli N, Reeves ND. Patellar Tendon adaptation in relation to load-intensity and contraction type. J Biomech. 2013;46:1893–9. https://doi.org/10.1016/j.jbiomech.2013.04.022.
Eriksen, C.S.; Svensson, R.B.; Gylling, A.T.; Couppé, C.; Magnusson, S.P.; Kjaer, M. Load magnitude affects patellar tendon mechanical properties but not collagen or collagen cross-linking after long-term strength training in older adults. BMC Geriatr 2019, 19. https://doi.org/10.1186/s12877-019-1043-0.
Franchi Mv, Atherton PJ, Reeves ND, Flück M, Williams J, Mitchell WK, Selby A, Beltran Valls RM, Narici Mv. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf). 2014;210:642–54. https://doi.org/10.1111/apha.12225.
Quinlan JI, Franchi MV, Gharahdaghi N, Badiali F, Francis S, Hale A, Phillips BE, Szewczyk N, Greenhaff PL, Smith K, et al. Muscle and tendon adaptations to moderate load eccentric vs. concentric resistance exercise in young and older males. Gerosci. 2021;43:567–1584. https://doi.org/10.1007/s11357-021-00396-0.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8. https://doi.org/10.1038/nprot.2008.73.
Babraj, JA.; Cuthbertson, D.J.R.; Smith, K.; Langberg, H.; Miller, B.; Krogsgaard, M.R.; Kjaer, M.; Rennie, M.J. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol - Endocrinol Metab 2005, 289. https://doi.org/10.1152/ajpendo.00243.2005.
Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Phillips BE, Szewczyk NJ, Greenhaff PL, Smith K, Atherton PJ. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J Physiol. 2016;594:7399–417. https://doi.org/10.1113/JP272857.
Reeves ND, Maganaris CN, Narici Mv. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548:971–81.
Mackey AL, Heinemeier KM, Koskinen SOA, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 2008;49:165–8. https://doi.org/10.1080/03008200802151672.
Gjaltema RAF, Bank RA. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit Rev Biochem Mol Biol. 2017;52:74–95.
Rappu P, Salo AM, Myllyharju J, Heino J. Role of prolyl hydroxylation in the molecular interactions of collagens. Essays Biochem. 2019;63:325–35.
Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–33. https://doi.org/10.1113/jphysiol.2005.093690.
Langberg H, Rosendal L, Kjær M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. https://doi.org/10.1111/j.1469-7793.2001.00297.x.
Franchi, M. v.; Wilkinson, D.J.; Quinlan, J.I.; Mitchell, W.K.; Lund, J.N.; Williams, J.P.; Reeves, N.D.; Smith, K.; Atherton, P.J.; Narici, M. v. Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle. Physiol Rep 2015, 3. https://doi.org/10.14814/phy2.12593.
Franchi, M. v.; Ruoss, S.; Valdivieso, P.; Mitchell, K.W.; Smith, K.; Atherton, P.J.; Narici, M. v.; Flück, M. Regional regulation of focal adhesion kinase after concentric and eccentric loading is related to remodelling of human skeletal muscle. Acta Physiola 2018, 223. https://doi.org/10.1111/apha.13056.
Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-β regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–11. https://doi.org/10.1006/abbi.1997.0102.
Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol. 1985;58:1572–6. https://doi.org/10.1152/jappl.1985.58.5.1572.
Koskinen SOA, Heinemeier KM, Olesen JL, Langberg H, Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol. 2004;96:861–4. https://doi.org/10.1152/japplphysiol.00489.2003.
Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP Modulates load-inducible IL-1β, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–62. https://doi.org/10.1002/jcb.10534.
Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, Narici Mv. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol. 2009;107:523–30. https://doi.org/10.1152/japplphysiol.00213.2009.
Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. 2007;191:111–21. https://doi.org/10.1111/j.1748-1716.2007.01714.x.
Wang JHC, Guo Q, Li B. Tendon biomechanics and mechanobiology - a minireview of basic concepts and recent advancements. J Hand Ther. 2012;25:133–41. https://doi.org/10.1016/j.jht.2011.07.004.
Vogel KG. What happens when tendons bend and twist? Proteoglycans J Musculoskelet Neuronal Interact. 2004;4:202–3.
Stenroth L, Peltonen J, Cronin NJ, Sipilä S, Finni T. Age-related differences in achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113:1537–44. https://doi.org/10.1152/japplphysiol.00782.2012.
Couppé C, Svensson RB, Grosset JF, Kovanen V, Nielsen RH, Olsen MR, Larsen JO, Praet SFE, Skovgaard D, Hansen M et al (2014) Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Omaha) 36. https://doi.org/10.1007/s11357-014-9665-9.
Zhou H, Wang SP, Herath K, Kasumov T, Sadygov RG, Previs SF, Kelley DE (2015) Tracer-based estimates of protein flux in cases of incomplete product renewal: evidence and implications of heterogeneity in collagen turnover. Am J Physiol Endocrinol Metab 309. https://doi.org/10.1152/ajpendo.00435.2014.
Abbott CB, Lawrence MM, Kobak KA, Lopes EBP, Peelor FF, Donald EJ, Van Remmen H, Griffin TM, Miller BF (2021) A novel stable isotope approach demonstrates surprising degree of age-related decline in skeletal muscle collagen proteostasis. Function 2. https://doi.org/10.1093/function/zqab028.
Busch R, Kim YK, Neese RA, Schade-Serin V. Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM et al (2006) Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochimica et Biophysica Acta - General Subjects 1760. https://doi.org/10.1016/j.bbagen.2005.12.023.
Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013;27:2074–9. https://doi.org/10.1096/fj.12-225599.
Acknowledgements
The authors would like to thank all the volunteers that took part in the study, as well as the clinical and technical support of the full clinical team.
Funding
This project was supported by the BBSRC [Grant number BB/K019104/1]. This work was also supported by the UK MRC (grant no. MR/P021220/1) as part of the MRC-ARUK Centre for Musculoskeletal Aging Research awarded to the Universities of Nottingham and Birmingham and supported by the National Institute of Health Research (NIHR) Nottingham Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health and Social Care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hannah Crossland and Matthew S. Brook are joint first author. Nathaniel J. Szewczyk, Kenneth Smith, Marco V. Narici and Philip J. Atherton are joint last author.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Crossland, H., Brook, M.S., Quinlan, J.I. et al. Metabolic and molecular responses of human patellar tendon to concentric- and eccentric-type exercise in youth and older age. GeroScience 45, 331–344 (2023). https://doi.org/10.1007/s11357-022-00636-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00636-x