Abstract
Objectives
To evaluate the potential of contrast-enhanced mammography (CEM) for reducing the biopsy rate of screening recalls.
Methods
Recalled women were prospectively enrolled to undergo CEM alongside standard assessment (SA) through additional views, tomosynthesis, and/or ultrasound. Exclusion criteria were symptoms, implants, allergy to contrast agents, renal failure, and pregnancy. SA and CEM were independently evaluated by one of six radiologists, who recommended biopsy or 2-year follow-up. Biopsy rates according to SA or recombined CEM (rCEM) were compared with the McNemar’s test. Diagnostic performance was calculated considering lesions with available final histopathology.
Results
Between January 2019 and July 2021, 220 women were enrolled, 207 of them (median age 56.6 years) with 225 suspicious findings analysed. Three of 207 patients (1.4%) developed mild self-limiting adverse reactions to iodinated contrast agent. Overall, 135/225 findings were referred for biopsy, 90/225 by both SA and rCEM, 41/225 by SA alone and 4/225 by rCEM alone (2/4 being one DCIS and one invasive carcinoma). The rCEM biopsy rate (94/225, 41.8%, 95% CI 35.5–48.3%) was 16.4% lower (p < 0.001) than the SA biopsy rate (131/225, 58.2%, 95% CI 51.7–64.5%). Considering the 124/135 biopsies with final histopathology (44 benign, 80 malignant), rCEM showed a 93.8% sensitivity (95% CI 86.2–97.3%) and a 65.9% specificity (95% CI 51.1–78.1%), all 5 false negatives being ductal carcinoma in situ detectable as suspicious calcifications on low-energy images.
Conclusions
Compared to SA, the rCEM-based work-up would have avoided biopsy for 37/225 (16.4%) suspicious findings. Including low-energy images in interpretation provided optimal overall CEM sensitivity.
Key Points
• The work-up of suspicious findings detected at mammographic breast cancer screening still leads to a high rate of unnecessary biopsies, involving between 2 and 6% of screened women.
• In 207 recalled women with 225 suspicious findings, recombined images of contrast-enhanced mammography (CEM) showed a 93.8% sensitivity and a 65.9% specificity, all 5 false negatives being ductal carcinoma in situ detectable on low-energy images as suspicious calcifications.
• CEM could represent an easily available one-stop shop option for the morphofunctional assessment of screening recalls, potentially reducing the biopsy rate by 16.4%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the benefits of mammographic screening outweigh its harms [1,2,3,4], various issues of the whole screening process are still unresolved [3]. Alongside a strong drive towards personalisation of screening strategies [5], research efforts are targeting a major drawback of mammographic screening, i.e. false positive recalls [3]. Indeed, even the current multi-layered imaging assessment still implies that women undergoing screening mammography have an estimated cumulative risk of undergoing a biopsy with a final benign outcome ranging between 2 and 6% [3, 6]. This figure is mirrored by the constantly high proportion of benign lesions (between 44 and 73%) reported in large-scale biopsy series [7,8,9,10].
Currently, the most employed assessment modalities—such as additional mammographic views, digital breast tomosynthesis, and ultrasound—rely exclusively on a morphologic appraisal of suspicious findings. Conversely, imaging techniques able to provide morphologic and functional information may foster a decrease in biopsy rates, i.e. an increase in the positive predictive value (PPV) of work-up examinations. This notion rests on the biological bases of functional assessment through contrast-enhanced examinations: tumour neoangiogenesis, resulting in leaky vessels that allow the entry of contrast agents into the interstitium, is a predominant feature of invasive cancers and more aggressive lesions [11, 12].
Among morpho-functional breast imaging techniques, contrast-enhanced mammography (CEM) could be better suited [13,14,15] than contrast-enhanced breast magnetic resonance imaging (CE-MRI) [16] for the work-up of screen-detected suspicious findings, as the latter has considerable contraindications, cost-related pitfalls, and may be suboptimal in assessing calcifications [17]. The potential of CEM has been highlighted also by a recent meta-analysis [18], where CEM had a 92% sensitivity and an 84% specificity when applied on mammography-detected suspicious findings.
CEM consists in a pair of mammograms (one low-energy, one high-energy) sequentially acquired after contrast agent administration and then recombined to minimize the appearance of unenhancing breast tissue, making enhanced areas recognizable [19]. Moreover, save from contrast administration, CEM is similar in workflow and time to a standard 4-view mammography or tomosynthesis [20], thus being much more tolerated, affordable, and available than CE-MRI [21,22,23,24].
The aim of this study was therefore to assess the potential of CEM for curtailing the biopsy rate in a prospectively enrolled population of women recalled for assessment of suspicious findings at screening mammography.
Methods
Study design and population
Approval for this bicentric prospective study was obtained by the Ethics Committee of IRCCS Ospedale San Raffaele, Milan, Italy (protocol code CESM; approved May 10th, 2018) and by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (protocol code P-20190076950, approved September 25th, 2019).
Enrolment in this study was proposed to all women aged 40–80 years referred to the Radiology Unit of IRCCS Policlinico San Donato, San Donato Milanese, Milan, Italy (Centre 1), or to the Department of Breast Radiology of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (Centre 2), for the work-up of suspicious findings detected at screening mammography (the structure and logistics of the local screening program being described in the Supplementary Material), between January 25th, 2019, and July 29th, 2021. Exclusion criteria were as follows: breast symptoms suspicious for breast cancer; pregnancy; presence of breast implants; allergy to iodinated contrast agents; renal failure (estimated glomerular filtration rate < 30 mL/min × 1.73 m2).
At both centres, standard assessment (SA) of suspicious findings was performed with additional mammographic views including mammographic magnification and/or spot compression, ultrasound, or digital breast tomosynthesis, according to the characteristics of each investigated suspicious finding.
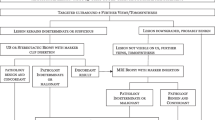
Eligible women willing to provide informed consent entered this study and, after collection of personal data (age, height, weight, menstrual cycle status), underwent CEM immediately after SA, as depicted in the protocol flowchart (Fig. E1).
Image acquisition and analysis
All CEM examinations were performed on a Senographe Pristina mammography system (GE Healthcare) at both centres. The following imaging protocol was used at both centres: 2 min before the first image acquisition, a 1.5 mL/kg dose of a non-ionic, monomeric, low-osmolar contrast agent (Iohexol 350 mgI/mL; GE Healthcare) was administered intravenously with an automated injector at a 2 mL/s flow rate, followed by a 30 mL saline flush. Then, standard mediolateral oblique and craniocaudal views were obtained in a maximum timeframe of 10 min, following the acquisition sequence commonly applied for diagnostic mammography at each centre [20]. All examinations times and the occurrence of any adverse reaction were recorded.
At each centre, two readers were involved in the interpretation of each patient’s examinations. The reader who performed the routine SA had no access to CEM; vice versa, CEM was independently interpreted by another reader, who was blinded to the results of the SA but aware of the mammographic findings that prompted the recall and had unrestricted access to the original mammographic images. Overall, six readers with a breast imaging experience ranging 6–30 years were involved in the interpretation process in the two centres.
SA results were categorised according to the BI-RADS classification [25] and women were either referred to biopsy or entered a 2-year follow-up with routine screening mammography and/or breast ultrasound. Conversely, since the reader interpreting CEM had access to the original mammographic images and CEM low-energy images are technically equivalent to a standard mammographic exam [26, 27] in providing a morphologic evaluation of the suspicious findings, CEM interpretation was focused on the recombined images (rCEM), in order to investigate the added value of the functional information provided by these contrast-enhanced images. On the basis of rCEM readings, the reader assessing CEM defined negative findings (i.e. those not needing a biopsy according to rCEM evaluation) and positive findings (those warranting a biopsy referral according to rCEM evaluation). If the reader interpreting CEM identified suspicious lesions different from those that prompted the recall and needing a dedicated work-up, the information was disclosed to the colleague performing SA and the work-up of these additional abnormalities was immediately performed according to the clinical practice currently used for additional findings at breast CE-MRI (targeted ultrasound, additional mammograms/tomosynthesis views, image-guided biopsy). Of note, as this design aims to evaluate the potential of rCEM to reduce the biopsy rate, CEM results could only be used to refer women to biopsy for suspicious findings that were not detectable at SA: biopsies recommended by SA were always performed, even with negative rCEM results.
Statistical analysis
The primary endpoint of this study was the potential rCEM biopsy rate, to be compared with the effectively performed SA biopsy rate, respectively calculated as
and
Secondary endpoints were as follows: (1) the number of adverse reactions to iodinated contrast agents (classified according to the 2021 American College of Radiology Manual [28]), and (2) SA and rCEM diagnostic performance, taking histopathology or 2-year follow-up as reference standard, considering in particular the number of detected and missed malignancies and, among them, of ductal carcinomas in situ (DCIS). For the latter secondary endpoint, we here present a subanalysis restricted to cases with available final histopathology reports, since the follow-up period is still ongoing.
Considering the presence of experienced breast radiologists at both centres and based on previous internal reviews of biopsy rates, we preliminarily assumed that women enrolled in this study would have a SA biopsy rate of about 50% and that rCEM could lead to about a 20% reduction in biopsy rate. We therefore calculated the sample size under the hypothesis of clinical superiority (i.e. of reducing the biopsy rate), assuming an 80% statistical power and a 5% α error. Under these assumptions, 197 women needed to be enrolled.
The Shapiro-Wilk test was used to perform distribution analysis. Consequentially, normal distributions were reported using mean ± standard deviation and non-normal distributions were reported as median with their interquartile range (IQR). The paired data comparison for the primary endpoint was performed with the McNemar’s test (p values < 0.05 considered statistically significant), while rates and diagnostic performance metrics for the secondary endpoints were determined along with their 95% confidence intervals (CIs). All analyses were performed with STATA, version MP 16.1 (StataCorp LLC).
Results
Between January 25, 2019, and July 29, 2021, 220 women were enrolled in this study, 122 at Centre 1 and 98 at Centre 2. CEM proved unfeasible in 3 of these 220 women (1.4%) because of contrast extravasation, while 10 other women were excluded from analysis after enrolment due to screening failure of exclusion criteria. The remaining 207 women who underwent both SA and CEM were included in the analysis: they had a median age of 56.6 years (IQR 50.1–65.3 years), 140/207 (67.6%) had already entered menopause, and 26/207 (12.6%) reported a family history of breast or ovarian cancer, no woman declaring to be a carrier of a genetic mutation increasing breast cancer risk. Out of 207 patients, 3 (1.4%) developed mild self-limiting adverse reactions to iodinated contrast agents, without the need of any medical intervention. The median CEM examination time was 4 min and 46 s (286 s, IQR 262–318 s).
The SA was prompted by a single suspicious finding in 191/207 women (92.3%), while in the remaining 16/207 women (7.7%) SA detected 2 suspicious findings (ipsilateral in 12 women, contralateral in 4 women). Of these 223 suspicious findings, 214 (95.9%) were already detectable on baseline mammography, 3/223 (1.4%) were suspicious axillary lymph nodes detected by ultrasonography, and the remaining 6/223 (2.7%) were inconclusive mammographic findings that were confirmed as suspicious by ultrasonography. Moreover, in 2 women (1.0%) rCEM identified an additional suspicious finding (both of them in the breast contralateral to the suspicious finding that prompted the recall).
As detailed in the study flowchart (Fig. 1), 225 suspicious findings were ultimately analysed for the assessment of the primary endpoint (Tables E1–E4): 131/225 were referred to biopsy by SA, for a SA biopsy rate of 58.2% (95% CI 51.7–64.5%), while 94/225 were referred to biopsy by rCEM, for a rCEM biopsy rate of 41.8% (95% CI 35.5–48.3%). Therefore, information from rCEM images would have engendered a 16.4% reduction in the biopsy rate, from 58.2 to 41.8% (p < 0.001). More specifically, SA and rCEM agreed on referring to biopsy 90/225 (40.0%) suspicious findings and agreed on sending to follow-up 90/225 (40.0%) suspicious findings. Conversely, rCEM would have spared the biopsy prompted by SA in 41/225 cases (18.2%) and effectively recommended biopsy for 4 findings (1.8%): 2 would have been sent to follow-up according to the SA, and 2 were rCEM-only detected findings. Thus, a biopsy was recommended either by SA or by rCEM for 135 suspicious findings. For 3 of them the procedure proved unfeasible, 2 other women elected to perform the recommended biopsy in other centres and were lost at follow-up, and 2 women—for whom CEM recommended a biopsy in contrast to the follow-up referral recommended by SA—refused to undergo the procedure.
Ultimately, 128 biopsies were performed at the two study centres, 75/128 (58.6%) under ultrasound guidance and 53/128 (41.4%) under stereotactic guidance. Overall, all 53 stereotactic-guided biopsies and 2 of the ultrasound-guided biopsies were performed as vacuum-assisted biopsies, while among the 73 remaining ultrasound-guided biopsies 68 (93.1%) were core-needle biopsies and 5 (6.9%) were fine-needle sampling. As detailed in Table 1, 42/128 biopsies had a benign result (32.8%) and 79/128 resulted in a diagnosis of malignancy (61.7%): DCIS accounted for 31.6% of malignancies (25/79). The remaining 7/128 biopsies (5.5%) had a B3 result: 4 cases were sent to imaging follow-up and were excluded from secondary endpoint analyses, while the other 3 were referred for surgery, 2 being downgraded to B2 lesions at surgical histopathology and one upgraded to a B5b lesion.
Thus, 124 lesions (44 benign and 80 malignant, 25 of which DCIS) had an available final histopathology report and were considered for the evaluation of the secondary endpoints related to diagnostic performance. Among the 122/124 lesions sent to biopsy by SA, 44 (36.1%) proved benign at histopathology, while the remaining 78 (63.9%) were classified as malignant, 24 of them being DCIS. The 2/124 suspicious findings that were not detected by SA but had a biopsy prompted by rCEM also resulted to be B5 lesions (one grade 2 DCIS and one invasive carcinoma of no special type). The sensitivity of SA (Table 2) was therefore 97.5% (95% CI 91.3–99.3%), with a PPV of 63.9% (95% CI 55.1–71.9%). Among the 90 suspicious findings sent to biopsy according to the information coming from rCEM images, 75/90 (83.3%, 20/90 DCIS) were malignant lesions (true positives, Fig. 2 and Fig. E2), while the remaining 15/90 (16.7%) were benign lesions (false positives, Fig. 3) Conversely, among the 34 biopsies with final reports that would have been spared by the evaluation of rCEM images (Table 3), histopathology revealed 29 benign (true negatives, Figs. 4 and 5) and 5 malignant lesions (false negatives, Fig. 6). Of note, all 5 were pure DCIS, i.e. without microinvasion (3 grade 2 and 2 grade 3): while none of them exhibited suspicious contrast enhancement on rCEM images, all were detectable on low-energy CEM images due to the presence of suspicious calcifications. Thus, while rCEM sensitivity (Table 4) was 93.8% (95% CI 86.2–97.3%), with a 65.9% specificity (95% CI 51.1–78.1%) and an 83.3% PPV (95% CI 74.3–89.6%), a combined reporting of rCEM images and low-energy images (focused on suspicious calcifications) to guide biopsy referral would have increased sensitivity to 100% (95% CI 95.4−100.0%).
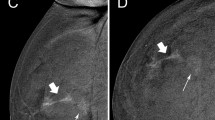
True positive case at contrast-enhanced mammography. A 53-year-old woman was recalled for suspicious calcifications in the left breast. An ultrasound-guided core needle biopsy was performed, resulting in a diagnosis of grade 2 ductal carcinoma in situ. Low-energy images (panels A and C) show multiple groups of pleomorphic calcifications in the left upper-outer quadrant (white arrows in light blue rectangles). Recombined images (panels B and D, light blue rectangles) revealed an area of non-mass enhancement involving the whole upper-outer quadrant
False positive case at contrast-enhanced mammography. A 69-year-old woman was recalled for a suspicious finding in the right breast, subsequently diagnosed as adenosis. Low-energy images (panels A and C) show a small opacity in the right upper-outer quadrant (light blue rectangles) with a correlated sub-centimetric enhancement focus on the recombined images (panels B and D, light blue rectangles)
True negative case at contrast-enhanced mammography. A 58-year-old woman was recalled for a suspicious retroareolar irregular opacity in the right breast (panel A and C, light blue rectangles). An ultrasound-guided core needle biopsy was performed, leading to a diagnosis of apocrine metaplasia. The absence of enhancement foci on recombined images (panels B and D) would have oriented the referral to follow-up
True negative case at contrast-enhanced mammography. A 49-year-old woman was recalled for a suspicious asymmetry in the upper quadrants of the left breast, not observable on the craniocaudal view (low-energy image, panel A) but definitely noticeable on the mediolateral oblique view (low-energy image, panel C, light blue rectangle). Standard assessment referred this finding to ultrasound-guided core needle biopsy, leading to a diagnosis of fibrosis. Conversely, the absence of enhancement in recombined images, both on the whole craniocaudal view (panel B) and in correspondence of the suspicious area on the mediolateral oblique view (panel D) would have oriented the work-up to a normal result with referral to re-screening at a 2-year interval
False negative case at contrast-enhanced mammography. A 67-year-old woman was recalled for a suspicious group of pleomorphic calcifications in the in the upper quadrants of the right breast, subsequently diagnosed as a grade 2 ductal carcinoma in situ, clearly visible on low-energy contrast-enhanced mammography images (panels A and C, light blue rectangles) but not associated with any enhancement on recombined images (panels B and D)
Discussion
Since the early days of CEM implementation, its use in the evaluation of abnormalities detected at screening mammography has been one of the most reported applications [14, 15]. Albeit with some caveats related to the contrast uptake of benign lesions [14, 15] and to equivocal enhancement conspicuity associated with calcifications clusters [29,30,31], retrospective studies have highlighted the potential of CEM to increase the PPV of the work-up process without compromising cancer detection [31,32,33,34,35]. We investigated this issue in a prospective setting, assessing the diagnostic gain granted by contrast-enhanced (rCEM) images, since low-energy CEM images—equivalent to standard mammograms [26, 27]—are also available in the SA process used as a comparator.
We observed a potential 16.4% net reduction of the biopsy rate that could be obtained by rCEM in the overall cohort of 225 suspicious findings, accompanied, in a subanalysis on 124 findings with final diagnosis, by a 19.4% PPV increase, in accordance with the multireader retrospective study by Zuley et al [35] on 60 BI-RADS 4 masses referred for biopsy. While their higher negative predictive value (98.3% versus our 85.3%) was likely prompted also by their exclusion of calcifications, we found similar, even though slightly higher, sensitivity (93.8% versus 90.3%) and specificity (65.9% versus 61.0%). Of note, we should consider that our specificity was negatively influenced by the exclusion of lesions referred for follow-up and will be recalculated after follow-up completion.
The biopsy increase solely attributable to CEM, i.e. the number of CEM-referred biopsies of suspicious findings that would have been sent to follow-up by SA plus the number of additional suspicious lesions detected by CEM but missed by screening mammography and SA, was 4/225 (1.8%). While the component of additional CEM-only findings (2/225, 0.9%) is of course lower than the 7.7% rate presented by Houben et al [34] in a study where screening mammography was the comparator instead of SA, we highlight that both cases in which the patient accepted to undergo the biopsy solely prompted by CEM were diagnosed as malignant lesions (one invasive carcinoma of no special type, one grade 2 DCIS), with a 100% PPV.
Importantly, DCIS presenting as calcifications clusters without associated contrast enhancement or with extremely faint enhancement were altogether responsible for the 6.2% drop in sensitivity of rCEM compared to the virtual 100% sensitivity of a combined reporting of low-energy images focused on suspicious calcifications and rCEM images, thus still supporting a direct biopsy referral of suspicious calcifications on the basis of their appearance on standard mammography or low-energy CEM images [31]. Without venturing in considerations about potential DCIS overdiagnosis [36], we however highlight that all were pure DCIS, without any microinvasion foci (3 intermediate grade, 2 high grade). As already reported [29,30,31], the negative predictive value of rCEM images for suspicious calcifications remains to be ascertained and, in our opinion, only large-scale dedicated studies will allow to solve this issue, especially also addressing DCIS overdiagnosis. Options in this direction involve the identification of characteristic enhancement patterns for cancers of low biological relevance [37] and the application of artificial intelligence–driven radiomic analysis [38]. The latter could be particularly useful considering how interpretation thresholds are influenced by the more equivocal visual conspicuity of lesion enhancement in rCEM images than in CE-MRI, compared to standard background parenchymal enhancement. In addition, only 3/207 patients (1.4%) developed mild self-limiting adverse reactions to iodinated contrast agent, confirming the CEM safety profile already reported in a meta-analysis [20].
Limitations of this study include—first—the only potential nature of the biopsy reduction we described and the non-randomised design: these characteristics prevented a clinical comparison of the SA and CEM-based work-up, also including patients’ preferences and cost-effectiveness, as will be done by the RACER trial [39]. Second, as already discussed for suspicious calcifications resulting in rCEM false negatives, our study design also factually oriented the analysis towards an appraisal of the contribution of rCEM information rather than of the “whole” CEM examination (low-energy and rCEM images). Finally, the ongoing follow-up period prevented us from exploring secondary endpoints related to diagnostic performance in the whole cohort, such as the correlation of imaging features with histopathology.
In conclusion, our study showed how a rCEM-based assessment of women recalled at first-level screening mammography is able to potentially engender a 16.4% reduction in biopsy rates compared to SA, maintaining high sensitivity (93.8%) with false negatives represented only by DCIS clearly detectable on low-energy CEM images. Coupled with the absence of moderate and severe adverse reactions to contrast agent, these data further highlight the role of CEM for the assessment of suspicious findings detected at screening mammography, avoiding a sizable number of unnecessary biopsies.
Change history
07 September 2022
Missing Open Access funding information has been added in the Funding Note.
Abbreviations
- CEM:
-
Contrast-enhanced mammography
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- CI:
-
Confidence interval
- DCIS:
-
Ductal carcinoma in situ
- IQR:
-
Interquartile interval
- PPV:
-
Positive predictive value
- rCEM:
-
Recombined contrast-enhanced mammography images
- SA:
-
Standard assessment
References
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M (2013) The benefits and harms of breast cancer screening: an independent review. Br J Cancer 108:2205–2240. https://doi.org/10.1038/bjc.2013.177
Nelson HD, Fu R, Cantor A et al (2016) Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164:244–255. https://doi.org/10.7326/M15-0969
Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L (2016) Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 164:256–267. https://doi.org/10.7326/M15-0970
Trimboli RM, Giorgi Rossi P, Battisti NML et al (2020) Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 11:105. https://doi.org/10.1186/s13244-020-00905-3
Pashayan N, Antoniou AC, Ivanus U et al (2020) Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol 17:687–705. https://doi.org/10.1038/s41571-020-0388-9
Hofvind S, Ponti A, Patnick J et al (2012) False-positive results in mammographic screening for breast cancer in Europe: a literature review and survey of service screening programmes. J Med Screen 19:57–66. https://doi.org/10.1258/jms.2012.012083
Andreu FJ, Sáez A, Sentís M et al (2007) Breast core biopsy reporting categories—an internal validation in a series of 3054 consecutive lesions. Breast 16:94–101. https://doi.org/10.1016/j.breast.2006.06.009
Youk JH, Kim E-K, Kim MJ, Oh KK (2008) Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol 190:202–207. https://doi.org/10.2214/AJR.07.2419
El-Sayed ME, Rakha EA, Reed J, Lee AH, Evans AJ, Ellis IO (2008) Audit of performance of needle core biopsy diagnoses of screen detected breast lesions. Eur J Cancer 44:2580–2586. https://doi.org/10.1016/j.ejca.2008.05.024
Jung I, Han K, Kim MJ et al (2020) Annual trends in ultrasonography-guided 14-gauge core needle biopsy for breast lesions. Korean J Radiol 21:259–267. https://doi.org/10.3348/kjr.2019.0695
Knopp MV, Weiss E, Sinn HP et al (1999) Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging 10:260–266. https://doi.org/10.1002/(SICI)1522-2586(199909)10:3<260::AID-JMRI6>3.0.CO;2-7
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407:249–257. https://doi.org/10.1038/35025220
Cozzi A, Schiaffino S, Sardanelli F (2019) The emerging role of contrast-enhanced mammography. Quant Imaging Med Surg 9:2012–2018. https://doi.org/10.21037/qims.2019.11.09
Jochelson MS, Lobbes MBI (2021) Contrast-enhanced mammography: state of the art. Radiology 299:36–48. https://doi.org/10.1148/radiol.2021201948
Neeter LMFH, Raat HPJF, Alcantara R et al (2021) Contrast-enhanced mammography: what the radiologist needs to know. BJR Open 3:20210034. https://doi.org/10.1259/bjro.20210034
Gommers JJ, Voogd AC, Broeders MJ et al (2021) Breast magnetic resonance imaging as a problem solving tool in women recalled at biennial screening mammography: a population-based study in the Netherlands. Breast 60:279–286. https://doi.org/10.1016/j.breast.2021.11.014
Bennani-Baiti B, Baltzer PA (2017) MR imaging for diagnosis of malignancy in mammographic microcalcifications: a systematic review and meta-analysis. Radiology 283:692–701. https://doi.org/10.1148/radiol.2016161106
Cozzi A, Magni V, Zanardo M, Schiaffino S, Sardanelli F (2022) Contrast-enhanced mammography: a systematic review and meta-analysis of diagnostic performance. Radiology 302:568–581. https://doi.org/10.1148/radiol.211412
Sensakovic WF, Carnahan MB, Czaplicki CD et al (2021) Contrast-enhanced mammography: how does it work? Radiographics 41:829–839. https://doi.org/10.1148/rg.2021200167
Zanardo M, Cozzi A, Trimboli RM et al (2019) Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 10:76. https://doi.org/10.1186/s13244-019-0756-0
Richter V, Hatterman V, Preibsch H et al (2018) Contrast-enhanced spectral mammography in patients with MRI contraindications. Acta Radiol 59:798–805. https://doi.org/10.1177/0284185117735561
Hobbs MM, Taylor DB, Buzynski S, Peake RE (2015) Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): patient preferences and tolerance. J Med Imaging Radiat Oncol 59:300–305. https://doi.org/10.1111/1754-9485.12296
Patel BK, Gray RJ, Pockaj BA (2017) Potential cost savings of contrast-enhanced digital mammography. AJR Am J Roentgenol 208:W231–W237. https://doi.org/10.2214/AJR.16.17239
Phillips J, Steinkeler J, Talati K et al (2018) Workflow considerations for incorporation of contrast-enhanced spectral mammography into a breast imaging practice. J Am Coll Radiol 15:881–885. https://doi.org/10.1016/j.jacr.2018.02.012
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA (2013) ACR BI-RADS® Atlas, breast imaging reporting and data system, 5th edn. American College of Radiology, Reston
Francescone MA, Jochelson MS, Dershaw DD et al (2014) Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 83:1350–1355. https://doi.org/10.1016/j.ejrad.2014.05.015
Lalji UC, Jeukens CRLPN, Houben I et al (2015) Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol 25:2813–2820. https://doi.org/10.1007/s00330-015-3695-2
ACR Committee on Drugs and Contrast Media (2021) ACR manual on contrast media. American College of Radiology, Reston
Cheung Y-C, Tsai H-P, Lo Y-F, Ueng S-H, Huang P-C, Chen S-C (2016) Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol 26:1082–1089. https://doi.org/10.1007/s00330-015-3904-z
Cheung Y-C, Juan Y-H, Lin Y-C et al (2016) Dual-energy contrast-enhanced spectral mammography: enhancement analysis on BI-RADS 4 non-mass microcalcifications in screened women. PLoS One 11:e0162740. https://doi.org/10.1371/journal.pone.0162740
Houben IP, Vanwetswinkel S, Kalia V et al (2019) Contrast-enhanced spectral mammography in the evaluation of breast suspicious calcifications: diagnostic accuracy and impact on surgical management. Acta Radiol 60:1110–1117. https://doi.org/10.1177/0284185118822639
Lobbes MBI, Lalji U, Houwers J et al (2014) Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 24:1668–1676. https://doi.org/10.1007/s00330-014-3154-5
Lalji UC, Houben IPLL, Prevos R et al (2016) Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 26:4371–4379. https://doi.org/10.1007/s00330-016-4336-0
Houben IPL, Van de Voorde P, Jeukens CRLPN et al (2017) Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur J Radiol 94:31–37. https://doi.org/10.1016/j.ejrad.2017.07.004
Zuley ML, Bandos AI, Abrams GS et al (2020) Contrast enhanced digital mammography (CEDM) helps to safely reduce benign breast biopsies for low to moderately suspicious soft tissue lesions. Acad Radiol 27:969–976. https://doi.org/10.1016/j.acra.2019.07.020
Grimm LJ, Rahbar H, Abdelmalak M, Hall AH, Ryser MD (2021) Ductal carcinoma in situ: state-of-the-art review. Radiology. https://doi.org/10.1148/radiol.211839
Cheung Y, Chen K, Yu C, Ueng S, Li C, Chen S (2021) Contrast-enhanced mammographic features of in situ and invasive ductal carcinoma manifesting microcalcifications only: help to predict underestimation? Cancers (Basel) 13:4371. https://doi.org/10.3390/cancers13174371
Marino MA, Pinker K, Leithner D et al (2020) Contrast-enhanced mammography and radiomics analysis for noninvasive breast cancer characterization: initial results. Mol Imaging Biol 22:780–787. https://doi.org/10.1007/s11307-019-01423-5
Neeter LMFH, Houben IPL, Nelemans PJ et al (2019) Rapid Access to Contrast-Enhanced spectral mammogRaphy in women recalled from breast cancer screening: the RACER trial study design. Trials 20:759. https://doi.org/10.1186/s13063-019-3867-5
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This study was supported by an unconditional research grant from GE Healthcare. This company did not have any influence on the study protocol planning, did not have any access to the study database, and was not involved in any way in the manuscript writing or submission phases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Francesco Sardanelli, MD.
Conflict of interest
Andrea Cozzi, Marianna Fanizza, and Veronica Magni controlled and analysed the data. None of them has relationships with any companies, whose products or services may be related to the subject matter of the article.
Simone Schiaffino received travel support from Bracco Imaging and is a member of the speakers’ bureau for GE Healthcare.
Francesco Sardanelli received research grants from — and is a member of the speakers’ bureau of — GE Healthcare, Bayer, and Bracco; he is also a member of the Bracco Advisory Group.
All other authors declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Andrea Cozzi and Giovanni Di Leo have significant statistical expertise.
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
This study was approved by the Ethics Committee of IRCCS Ospedale San Raffaele, Milano, Italy (protocol code CESM; approved on May 10, 2018), and by the Ethics Committee of Fondazione IRCCS Policlinico San Matteo, Pavia, Italy (protocol code P-20190076950, approved on September 25, 2019).
Study subjects or cohorts overlap
One-hundred twenty patients included in this study were previously reported in another article (doi: 10.3390/cancers14071774) that is however solely focused on the analysis of the radiation dose of CEM and does not involve any kind of consideration or analysis related to the topic of the present study.
Methodology
• Prospective
• Diagnostic
• Multicentre
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2.23 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cozzi, A., Schiaffino, S., Fanizza, M. et al. Contrast-enhanced mammography for the assessment of screening recalls: a two-centre study. Eur Radiol 32, 7388–7399 (2022). https://doi.org/10.1007/s00330-022-08868-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08868-3