Abstract
Introduction
Ocrelizumab, an antiCD-20 antibody, is the only drug approved to treat patients with primary progressive multiple sclerosis (pwPPMS). Not all candidates receive this treatment due to prescription limitations. Rituximab, another antiCD-20 antibody, has been used off-label in pwPPMS before and after ocrelizumab approval. However, studies comparing effectiveness of both drugs are lacking.
Objective
To evaluate effectiveness of rituximab and ocrelizumab in pwPPMS under real-life conditions.
Methods
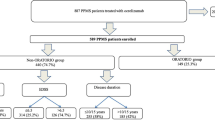
We conducted a multicentric observational study of pwPPMS that started ocrelizumab or rituximab according to clinical practice, with a minimum follow-up of 1 year. Data was collected prospectively and retrospectively. Primary outcome was time to confirmed disability progression at 3 months (CDW). Secondary outcome was serum neurofilament light chain levels (sNFL) at the end of follow-up.
Results
95 out 111 pwPPMS fulfilled inclusion criteria and follow-up data availability: 49 (51.6%) received rituximab and 46 (48.4%) ocrelizumab. Rituximab-treated patients had significantly higher baseline EDSS, disease duration and history of previous disease-modifying treatment (DMT) than ocrelizumab-treated patients. After a mean follow-up of 18.3 months (SD 5.9), 26 patients experienced CDW (21.4%); 15 (30.6%) in the rituximab group; and 11 (23.9%) in the ocrelizumab group. Survival analysis revealed no differences in time to CDW. sNFL were measured in 60 patients and no differences between groups were found.
Interpretation
We provide real-world evidence of effectiveness of ocrelizumab and rituximab in pwPPMS. No differences in time to CDW were found between treatments. However, this study cannot establish equivalence of treatments and warrant clinical trial to confirm our findings.


Similar content being viewed by others
References
Hauser SL, Waubant E, Arnold DL et al (2008) B cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358:676–688. https://doi.org/10.1056/NEJMOA0706383
Bar-Or A, Calabresi PAJ, Arnlod D et al (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63:395–400. https://doi.org/10.1002/ANA.21363
Naismith RT, Piccio L, Lyons JA et al (2010) Rituximab add-on therapy for breakthrough relapsing multiple sclerosis. Neurology 74:1860–1867. https://doi.org/10.1212/WNL.0B013E3181E24373
Ocrevus | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/ocrevus. Accessed 7 Nov 2021
Therapeutic positioning report of ocrelizumab (Ocrevus ®) in multiple sclerosis , January 15 ,2019 (v.1). In: Spanish Agency Med. Med. Devices. https://www.aemps.gob.es/medicamentos-de-uso-humano/informes-de-posicionamiento-terapeutico/#L04-Inmunosupresores
Thebault S, Bose G, Booth R, Freedman MS (2021) Serum neurofilament light in MS: The first true blood-based biomarker? Mult Scler J
FDA approves new drug to treat multiple sclerosis | FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treat-multiple-sclerosis. Accessed 7 Nov 2021
Boyer-Suavet S, Andreani M, Lateb M et al (2020) Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. https://doi.org/10.3389/FIMMU.2019.03069
Schuh E, Berer K, Mulazzani M et al (2016) Features of human CD3 + CD20 + T cells. J Immunol 197:1111–1117. https://doi.org/10.4049/JIMMUNOL.1600089/-/DCSUPPLEMENTAL
Gingele S, Skripuletz T, Jacobs R (2020) Role of CD20+ T cells in multiple sclerosis: implications for treatment with ocrelizumab. Neural Regen Res 15:663. https://doi.org/10.4103/1673-5374.266913
Klein C, Lammens A, Schäfer W et al (2012) Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs 5:22–33. https://doi.org/10.4161/MABS.22771
Morschhauser F, Marlton P, Vitolo U et al (2010) Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol 21:1870–1876. https://doi.org/10.1093/ANNONC/MDQ027
Petereit HF, Rubbert-Roth A (2009) Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler 15:189–192. https://doi.org/10.1177/1352458508098268
Kamburova EG, Koenen HJPM, Borgman KJE et al (2013) A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 13:1503–1511. https://doi.org/10.1111/AJT.12220
Brown POPR Ocrelizumab Pharmacology Review FDA.gov. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761053Orig1s000PharmR.pdf
Bell L, Lenhart A, Rosenwald A et al (2020) Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front Immunol 10:3090. https://doi.org/10.3389/FIMMU.2019.03090/BIBTEX
ISRCTN—ISRCTN16295177: A study to investigate the safety, tolerability, and processing by the body of intravenous RO7121932 in patients with multiple sclerosis. https://www.isrctn.com/ISRCTN16295177. Accessed 12 Jan 2022
Martin MDP, Cravens PD, Winger R et al (2009) Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol 66:1016–1020. https://doi.org/10.1001/ARCHNEUROL.2009.157
Cross AH, Stark JL, Lauber J et al (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180:63–70. https://doi.org/10.1016/J.JNEUROIM.2006.06.029
Piccio L, Naismith RT, Trinkaus K et al (2010) Changes in B- and T lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol 67:707–714. https://doi.org/10.1001/ARCHNEUROL.2010.99
Tobias D et al. 279399; 65 (2019) Serum immunoglobulin levels and risk of serious infections in the.... ECTRIMS Online Library. Derfuss T. Sep 11 2019; 279399. In: ECTRIMS Online Library
Londoño AC, Mora CA (2018) Role of CXCL13 in the formation of the meningeal tertiary lymphoid organ in multiple sclerosis. F1000Research 7:514. https://doi.org/10.1288/F1000RESEARCH.14556.3
Bergman J, Burman J, Gilthorpe JD et al (2018) Intrathecal treatment trial of rituximab in progressive MS. Neurology 91:e1893–e1901. https://doi.org/10.1212/WNL.0000000000006500
Komori M, Lin YC, Cortese I et al (2016) Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol 3:166. https://doi.org/10.1002/ACN3.293
Ahdab R, Creange A, Benaderette S, Lefaucheur J-P (2009) Cervical spondylotic amyotrophy presenting as dropped head syndrome. Clin Neurol Neurosurg 111:874–876. https://doi.org/10.1016/j.clineuro.2009.07.005
von Büdingen H-C, Bischof A, Eggers EL et al (2017) Onset of secondary progressive MS after long-term rituximab therapy—a case report. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.377
Brand RM, Friedrich V, Diddens J et al (2021) Anti-CD20 depletes meningeal B cells but does not halt the formation of meningeal ectopic lymphoid tissue. Neurol - Neuroimmunol Neuroinflammation. https://doi.org/10.1212/NXI.0000000000001012
Funding
This work has been supported by a grant from the Health Institute Carlos III (PI20/01446) and FEDER funding.
Author information
Authors and Affiliations
Contributions
CA and BC for conception and design of the study, acquisition and analysis of data and critical revision of the manuscript. CQ-B por drafting the manuscript, acquisition and analysis of data. FG, APS, LN, MC, LL, JM, EC, AB, SC, JAD, FCP-M, SG-P, RG, LC, JC for acquisition of data and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
B Casanova has received consulting fees form Merck, Sanofi-Genzyme, Biogen-Idec, Novartis, and Roche. AP Sempere has received consulting and speaking fees from Biogen, Merck Serono, Novartis, Teva and Roche. F Gascon has received consulting fees and grants for travel from Teva, Merck-Serono, Biogen, Bayer, Novartis, Sanofi-Genzyme, Almirall and Roche. L Navarro has received consulting and speaking fees from Sanofi-Genzyme, Merk, Biogen-Idec and Roche.
Ethical standard statement
The study was conducted in accordance with local ethical standards and approved by the ethics committee.
Rights and permissions
About this article
Cite this article
Alcalá, C., Quintanilla-Bordás, C., Gascón, F. et al. Effectiveness of rituximab vs. ocrelizumab for the treatment of primary progressive multiple sclerosis: a real-world observational study. J Neurol 269, 3676–3681 (2022). https://doi.org/10.1007/s00415-022-10989-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-10989-0