Abstract
Purpose
The significantly higher incidence of aneurysms in patients with arteriovenous malformations (AVMs) suggests a strong hemodynamic relationship between these lesions. The presence of an AVM alters hemodynamics in proximal vessels by drastically changing the distal resistance, thus affecting intra-aneurysmal flow. This study discusses the challenges associated with patient-specific modeling of aneurysms in the presence of AVMs.
Methods
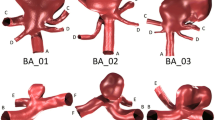
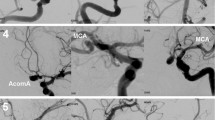
We explore how the presence of a generic distal AVM affects upstream aneurysms by examining the relationship between distal resistance and aneurysmal wall shear stress using physiologically realistic estimates for the influence of the AVM on hemodynamics. Using image-based computational models of aneurysms and surrounding vasculature, aneurysmal wall-shear stress is calculated for a range of distal resistances corresponding to the presence of AVMs of various sizes and compared with a control case representing the absence of an AVM.
Results
In the patient cases considered, the alteration in aneurysmal wall shear stress due to the presence of an AVM is considerable, as much as 19 times the base case wall shear stress. Furthermore, the relationship between aneurysmal wall shear stress and distal resistance is shown to be highly geometry-dependent and nonlinear. In most cases, the range of physiologically realistic possibilities for AVM-related distal resistance are so large that patient-specific flow measurements are necessary for meaningful predictions of wall shear stress.
Conclusions
The presented work offers insight on the impact of distal AVMs on aneurysmal wall shear stress using physiologically realistic computational models. Patient-specific modeling of hemodynamics in aneurysms and associated AVMs has great potential for understanding lesion pathogenesis, surgical planning, and assessing the effect of treatment of one lesion relative to another. However, we show that modeling approaches cannot usually meaningfully quantify the impact of AVMs if based solely on imaging data from CT and X-ray angiography, currently used in clinical practice. Based on recent studies, it appears that 4D flow MRI is one promising approach to obtaining meaningful patient-specific flow boundary conditions that improve modeling fidelity.




Similar content being viewed by others
References
Ansari, S., S. Schnell, T. Carroll, P. Vakil, M. Hurley, C. Wu, J. Carr, B. Bendok, H. Batjer, and M. Markl. Intracranial 4D flow MRI: toward individualized assessment of arteriovenous malformation hemodynamics and treatment-induced changes. Am. J. Neuroradiol. 34(10):1922, 2013. https://doi.org/10.3174/ajnr.A3537.
Aristova, M., A. Vali, S. A. Ansari, A. Shaibani, T. D. Alden, M. C. Hurley, B. S. Jahromi, M. B. Potts, M. Markl, and S. Schnell. Standardized evaluation of cerebral arteriovenous malformations using flow distribution network graphs and dual-venc 4D flow MRI. J. Magn. Reson. Imaging. 50(6):1718, 2019. https://doi.org/10.1002/jmri.26784.
Berg, P., S. Voß, S. Saalfeld, G. Janiga, A. W. Bergersen, K. Valen-Sendstad, J. Bruening, L. Goubergrits, A. Spuler, N. M. Cancelliere, D. A. Steinman, V. M. Pereira, T. L. Chiu, A. C. O. Tsang, B. J. Chung, J. R. Cebral, S. Cito, J. Pallarès, G. Copelli, B. Csippa, G. Paál, S. Fujimura, H. Takao, S. Hodis, G. Hille, C. Karmonik, S. Elias, K. Kellermann, M. O. Khan, A. L. Marsden, H. G. Morales, S. Piskin, E. A. Finol, M. Pravdivtseva, H. Rajabzadeh-Oghaz, N. Paliwal, H. Meng, S. Seshadhri, M. Howard, M. Shojima, S. I. Sugiyama, K. Niizuma, S. Sindeev, S. Frolov, T. Wagner, A. Brawanski, Y. Qian, Y. A. Wu, K. D. Carlson, D. Dragomir-Daescu, and O. Beuing. Multiple aneurysms anatomy challenge 2018 (MATCH): phase I: segmentation. Cardiovasc. Eng. Technol. 9(4):565, 2018. https://doi.org/10.1007/s13239-018-00376-0.
Boussel, L., V. Rayz, A. Martin, G. Acevedo-Bolton, M. T. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Phase-Contrast MRI measurements in intra-cranial aneurysms in-vivo of flow patterns, velocity fields and wall shear stress: a comparison with CFD. Magn. Resonan. Med. 61(2):409, 2009. https://doi.org/10.1002/mrm.21861.
Boussel, L., V. Rayz, C. McCulloch, A. Martin, G. Acevedo-Bolton, M. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Aneurysm growth occurs at region of low wall shear stress: patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke. 39(11):2997, 2008. https://doi.org/10.1161/STROKEAHA.108.521617.
Brown, R. D., D. O. Wiebers, and G. S. Forbes. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J. Neurosurg. 73(6):859, 1990. https://doi.org/10.3171/jns.1990.73.6.0859.
Cebral, J., E. Ollikainen, B. Chung, F. Mut, V. Sippola, B. Jahromi, R. Tulamo, J. Hernesniemi, M. Niemelä, A. Robertson, and J. Frösen. Flow conditions in the intracranial aneurysm lumen are associated with inflammation and degenerative changes of the aneurysm wall. Am. J. Neuroradiol. 38(1):119, 2017. https://doi.org/10.3174/ajnr.A4951.
Chang, W., M. W. Loecher, Y. Wu, D. B. Niemann, B. Ciske, B. Aagaard-Kienitz, S. Kecskemeti, K. M. Johnson, O. Wieben, C. Mistretta, and P. Turski. Hemodynamic changes in patients with arteriovenous malformations assessed using high-resolution 3D radial phase-contrast MR angiography. Am. J. Neuroradiol. 33(8):1565, 2012. https://doi.org/10.3174/ajnr.A3010.
Detmer, F. J., B. J. Chung, F. Mut, M. Slawski, F. Hamzei-Sichani, C. Putman, C. Jiménez, and J. R. Cebral. Development and internal validation of an aneurysm rupture probability model based on patient characteristics and aneurysm location, morphology, and hemodynamics. Int. J. Comput. Assist. Radiol. Surg. 13(11):1767, 2008. https://doi.org/10.1007/s11548-018-1837-0.
Duckwiler, G., J. Dion, F. Vinuela, B. Jabour, N. Martin, and J. Bentson. Intravascular microcatheter pressure monitoring: experimental results and early clinical evaluation. Am. J. Neuroradiol. 11(1):169, 1990.
Esmaily Moghadam, M., Y. Bazilevs, T. Y. Hsia, I. E. Vignon-Clementel, and A. L. Marsden. A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Comput. Mech. 48(3):277, 2011. https://doi.org/10.1007/s00466-011-0599-0.
Esmaily Moghadam, M., I. E. Vignon-Clementel, R. Figliola, and A. L. Marsden. A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J. Comput. Phys. 244:63, 2013. https://doi.org/10.1016/j.jcp.2012.07.035.
Fogarty-Mack, P., J. Pile-Spellman, L. Hacein-Bey, A. Osipov, J. DeMeritt, E. C. Jackson, and W. L. Young. The effect of arteriovenous malformations on the distribution of intracerebral arterial pressures. Am. J. Neuroradiol. 17(8):1443, 1996.
Francis, C., L. Frederic, L. Sylvie, P. Prasanna, and D. Henri. Scaling laws for branching vessels of human cerebral cortex. Microcirculation. 16(4):331, 2009. https://doi.org/10.1080/10739680802662607.
Frösen, J., J. Cebral, A. M. Robertson, and T. Aoki. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg. Focus. 47(1):E21, 2019. https://doi.org/10.3171/2019.5.FOCUS19234.
Gao, E., W. L. Young, E. Ornstein, J. Pile-Spellman, and M. Qiyuan. A theoretical model of cerebral hemodynamics: application to the study of arteriovenous malformations. J. Cereb. Blood Flow Metab. 17(8):905, 1997. https://doi.org/10.1097/00004647-199708000-00009.
Gao, E., W. L. Young, J. Pile-Spellman, S. Joshi, H. Duong, P. E. Stieg, and Q. Ma. Cerebral arteriovenous malformation feeding artery aneurysms: a theoretical model of intravascular pressure changes after treatment. Neurosurgery. 41(6):1345, 1997. https://doi.org/10.1097/00006123-199712000-00020.
Jungreis, C. A., and J. A. Horton. Pressure changes in the arterial feeder to a cerebral AVM as a guide to monitoring therapeutic embolization. Am. J. Neuroradiol. 10(5):1057, 1989.
Jungreis, C. A., J. A. Horton, and S. T. Hecht. Blood pressure changes in feeders to cerebral arteriovenous malformations during therapeutic embolization. Am. J. Neuroradiol. 10(3):575, 1989.
Lan, H., A. Updegrove, N. M. Wilson, G. D. Maher, S. C. Shadden, and A. L. Marsden. A re-engineered software interface and workflow for the open-source simvascular cardiovascular modeling package. J. Biomech. Eng.140(2):245011, 2018. https://doi.org/10.1115/1.4038751.
Levitt, M., P. McGah, A. Aliseda, P. Mourad, J. Nerva, S. Vaidya, R. Morton, B. Ghodke, and L. Kim. Cerebral aneurysms treated with flow-diverting stents: computational models with intravascular blood flow measurements. Am. J. Neuroradiol. 35(1):143, 2014. https://doi.org/10.3174/ajnr.A3624.
MacDonald, M. E., and R. Frayne. Phase contrast MR imaging measurements of blood flow in healthy human cerebral vessel segments. Physiol. Meas. 36(7):1517, 2015. https://doi.org/10.1088/0967-3334/36/7/1517.
Markl, M., A. Frydrychowicz, S. Kozerke, M. Hope, and O. Wieben. 4D flow MRI. J. Magn. Reson. Imaging. 36(5):1015, 2012. https://doi.org/10.1002/jmri.23632.
Marsden, A. L., and M. Esmaily-Moghadam. Multiscale modeling of cardiovascular flows for clinical decision support. Appl. Mech. Rev. 67(3):030804, 2015. https://doi.org/10.1115/1.4029909.
McElroy, M., and A. Keshmiri. Impact of using conventional inlet/outlet boundary conditions on haemodynamic metrics in a subject-specific rabbit aorta. Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 232(2):103, 2018. https://doi.org/10.1177/0954411917699237.
Meng, H., V. M. Tutino, J. Xiang, and A. Siddiqui. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. Am. J. Neuroradiol. 35(7):1254, 2014. https://doi.org/10.3174/ajnr.A3558.
Nornes, H., and A. Grip. Steal and cerebral arteriovenous malformations. J. Neurosurg. 53(4):456, 1980. https://doi.org/10.3171/jns.1980.53.4.0456.
Rammos, S. K., B. Gardenghi, C. Bortolotti, H. J. Cloft, and G. Lanzino. Aneurysms associated with brain arteriovenous malformations. Am. J. Neuroradiol. 37(11):1966, 2016. https://doi.org/10.3174/ajnr.A4869.
Rayz, V. L., A. Abla, L. Boussel, J. R. Leach, G. Acevedo-Bolton, D. Saloner, and M. T. Lawton. Computational modeling of flow-altering surgeries in basilar aneurysms. Ann. Biomed. Eng. 43(5):1210, 2015. https://doi.org/10.1007/s10439-014-1170-x.
Rayz, V. L., L. Boussel, L. Ge, J. R. Leach, A. J. Martin, M. T. Lawton, C. McCulloch, and D. Saloner. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Ann. Biomed. Eng. 38(10):3058, 2010. https://doi.org/10.1007/s10439-010-0065-8.
Rayz, V., L. Boussel, M. Lawton, G. Acevedo-Bolton, L. Ge, W. Young, R. Higashida, and D. Saloner. Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann. Biomed. Eng. 36(11):1793, 2008. https://doi.org/10.1007/s10439-008-9561-5.
Rayz, V. L., M. T. Lawton, A. J. Martin, W. L. Young, and D. Saloner. Numerical simulation of pre-and postsurgical flow in a giant basilar aneurysm. J. Biomech. Eng. 130(2):021004, 2008. https://doi.org/10.1115/1.2898833.
Redekop, G., K. TerBrugge, W. Montanera, and R. Willinsky. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J. Neurosurg. 89(4):539, 1998. https://doi.org/10.3171/jns.1998.89.4.0539.
Rossitti, S., and J. Löfgren. Vascular dimensions of the cerebral arteries follow the principle of minimum work. Stroke. 24(3):371, 1993. https://doi.org/10.1161/01.STR.24.3.371.
Seo, J., D. E. Schiavazzi, A. M. Kahn, and A. L. Marsden. The effects of clinically-derived parametric data uncertainty in patient-specific coronary simulations with deformable walls. Int. J. Numer. Methods Biomed. Eng.36(8):e3351, 2020. https://doi.org/10.1002/cnm.3351.
Shakur, S. F., S. Amin-Hanjani, M. Abouelleil, V. A. Aletich, F. T. Charbel, and A. Alaraj. Changes in pulsatility and resistance indices of cerebral arteriovenous malformation feeder arteries after embolization and surgery. Neurol. Res. 39(1):7, 2017. https://doi.org/10.1080/01616412.2016.1258970.
Shakur, S. F., S. Amin-Hanjani, H. Mostafa, F. T. Charbel, and A. Alaraj. Hemodynamic characteristics of cerebral arteriovenous malformation feeder vessels with and without aneurysms. Stroke. 46(7):1997, 2015. https://doi.org/10.1161/STROKEAHA.115.009545.
Spetzler, R. F., R. W. Hargraves, P. W. McCormick, J. M. Zabramski, R. A. Flom, and R. S. Zimmerman. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J. Neurosurg. 76(6):918, 1992. https://doi.org/10.3171/jns.1992.76.6.0918.
Sturdy, J., J. K. Kjernlie, H. M. Nydal, V. G. Eck, and L. R. Hellevik. Uncertainty quantification of computational coronary stenosis assessment and model based mitigation of image resolution limitations. J. Comput. Sci. 31:137, 2019. https://doi.org/10.1016/j.jocs.2019.01.004.
Tanaka, M. In Brain Arteriovenous Malformations: Pathogenesis, Epidemiology, Diagnosis, Treatment and Outcome, edited by V. Benes and O. Bradac. Cham: Springer International Publishing, 2017, pp. 5–22. https://doi.org/10.1007/978-3-319-63964-2_2.
Taylor, C. A., T. A. Fonte, and J. K. Min. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J. Am. Coll. Cardiol. 61(22):2233, 2013. https://doi.org/10.1016/j.jacc.2012.11.083.
Updegrove, A., N. M. Wilson, J. Merkow, H. Lan, A. L. Marsden, and S. C. Shadden. SimVascular: an open source pipeline for cardiovascular simulation. Ann. Biomed. Eng. 45(3):525, 2017. https://doi.org/10.1007/s10439-016-1762-8.
Vali, A., A. A. Abla, M. T. Lawton, D. Saloner, and V. L. Rayz. Computational fluid dynamics modeling of contrast transport in basilar aneurysms following flow-altering surgeries. J. Biomech. 50:195, 2017. https://doi.org/10.1016/j.jbiomech.2016.11.028.
van der Giessen, A. G., H. C. Groen, P. A. Doriot, P. J. de Feyter, A. F. van der Steen, F. N. van de Vosse, J. J. Wentzel, and F. J. Gijsen. The influence of boundary conditions on wall shear stress distribution in patients specific coronary trees. J. Biomech. 44(6):1089, 2011. https://doi.org/10.1016/j.jbiomech.2011.01.036.
Whiting, C. H., and K. E. Jansen. A stabilized finite element method for the incompressible Navier-Stokes equations using a hierarchical basis. Int. J. Numer. Methods Fluids. 35(1):93, 2001.
Yushkevich, P. A., J. Piven, H. C. Hazlett, R. G. Smith, S. Ho, J. C. Gee, and G. Gerig. User-guided level set segmentation of anatomical structures with ITK-SNAP. NeuroImage. 31(3):1116, 2006. https://doi.org/10.1016/j.neuroimage.2006.01.015.
Zarrinkoob, L., K. Ambarki, A. Wåhlin, R. Birgander, A. Eklund, and J. Malm. Blood flow distribution in cerebral arteries. J. Cereb. Blood Flow Metab. 35(4):648, 2015. https://doi.org/10.1038/jcbfm.2014.241.
Zhang, J., S. Bhattacharya, and P. P. Vlachos. Using uncertainty to improve pressure field reconstruction from PIV/PTV flow measurements. Exp. Fluids. 61(6):131, 2020. https://doi.org/10.1007/s00348-020-02974-y.
Zhao, M., S. Amin-Hanjani, S. Ruland, A. Curcio, L. Ostergren, and F. Charbel. Regional cerebral blood flow using quantitative MR angiography. Am. J. Neuroradiol. 28(8):1470, 2007. https://doi.org/10.3174/ajnr.A0582.
Zhou, Y., G. S. Kassab, and S. Molloi. On the design of the coronary arterial tree: a generalization of Murray’s law. Phys. Med. Biol. 44(12):2929, 1999. https://doi.org/10.1088/0031-9155/44/12/306.
Zhou, G., Y. Zhu, Y. Yin, M. Su, and M. Li. Association of wall shear stress with intracranial aneurysm rupture: systematic review and meta-analysis. Sci. Rep. 7(1):5331, 2017. https://doi.org/10.1038/s41598-017-05886-w.
Acknowledgments
K.A.S.B. was supported in part by the Lillian Gilbreth Postdoctoral Fellowship from Purdue’s College of Engineering. K.A.S.B. and I.C.C. additionally received partial support from the US National Science Foundation under grant No. CBET-1705637.
Conflict of interest
Kimberly Boster, Tanmay Shidhore, Aaron Cohen-Gadol, Ivan Christov, and Vitaliy Rayz declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boster, K.A.S., Shidhore, T.C., Cohen-Gadol, A.A. et al. Challenges in Modeling Hemodynamics in Cerebral Aneurysms Related to Arteriovenous Malformations. Cardiovasc Eng Tech 13, 673–684 (2022). https://doi.org/10.1007/s13239-022-00609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-022-00609-3