Abstract
Purpose
“Non thyroidal illness syndrome” (NTIS) or “euthyroid sick syndrome” (ESS) is a possible biochemical finding in euthyroid patients with severe diseases. It is characterized by a reduction of serum T3 (fT3), sometimes followed by reduction of serum T4 (fT4). The relationship between thyroid hormones levels and mortality is well known and different studies showed a direct association between NTIS and mortality. The sudden spread of the 2019 novel coronavirus (SARS-CoV 2) infection (COVID-19) and its high mortality become a world healthcare problem. Our aim in this paper was to investigate if patients affected by COVID-19 presented NTIS and the relationship between thyroid function and severity of this infection.
Methods
We evaluated the thyroid function in two different groups of consecutive patients affected by COVID-19 with respect to a control group of euthyroid patients. Group A included patients hospitalized for COVID-19 pneumonia while patients requiring intensive care unit (ICU) for acute respiratory syndrome formed the group B. Group C identified the control group of euthyroid patients.
Results
Patients from group A and group B showed a statistically significant reduction in fT3 and TSH compared to group C. In group B, compared to group A, a further statistically significant reduction of fT3 and TSH was found.
Conclusions
COVID-19 in-patients can present NTIS. FT3 and TSH serum levels are lower in patients with more severe symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alterations in thyroid hormones levels during critical illness describe the “non-thyroidal illness syndrome” (NTIS) or “euthyroid sick syndrome” (ESS). The typical hormonal pictures are characterized by the reduction of serum T3 (fT3) levels, but throughout deterioration of general condition, T4 (fT4) values may decrease as well, producing a combined low fT3-low fT4 state [1, 2]. This condition does not lead to a compensatory increase of TSH levels; on the contrary, the TSH levels decrease [3,4,5]. Considering the relationship between thyroid hormones levels and mortality, different studies showed an association between NTIS and mortality [1, 2]. Indeed, in the intensive care unit (ICU) more than 70% of the patients show low T3 levels [6].
During 2019–2020, the whole world went through the 2019 novel coronavirus (SARS-CoV 2) pandemic. The sudden spread of SARS-CoV 2, originated from a zoonotic transmission, has become a huge healthcare problem worldwide, especially for its contagiousness. Patients affected by SARS-CoV 2 infection (COVID-19) can show different clinical presentations: from mild symptoms to a severe hypoxic respiratory failure caused by an important alteration of ventilation/perfusion ratio [7]. Lung involvement at CT scan is characterized by typical features such as the presence of dense lesions in multiple lung lobes, ground-glass opacity with consolidation shadows or cord-like shadows. If a rapid deterioration of hypoxemia occurs, intubation with invasive mechanical ventilation is mandatory and patients are moved to ICU department. In the present study we evaluated the thyroid function in two groups of COVID-19 patients, with respect to a control group of euthyroid patients. The two groups of COVID-19 patients differed in the severity of the clinical picture, thus a comparison between the two groups of COVID-19 patients was evaluated as well.
Patients and methods
We retrospectively evaluated 46 consecutive COVID-19 patients’ records, after the exclusion of patients with a positive medical history for hypo- or hyperthyroidism, thyroiditis or in treatment with lithium and/or amiodarone.
The patients were 14 females and 32 males, all of them were hospitalized between February 1 and February 29, 2020 at the “Lazzaro Spallanzani” National Institute for Infectious Diseases, Rome, Italy. The diagnosis of COVID-19 pneumonia was based on the presence of suggestive symptoms and consistent radiological imaging at chest CT scan and SARS-CoV 2 detection by real time PCR (RT-PCR) at nasopharyngeal swab [8]. Two groups of patients were evaluated for thyroid function: in-patients for COVID-19 pneumonia (group A, n = 23) aged 60.8 ± 17.0, 35–85 years (mean ± SD, range) and ICU patients (group B, n = 23) aged 58.4 ± 12.1, 31–80 years. A third group (group C) with normal thyroid function (n = 20) has been included as a control group (5 females and 15 males), aged 59.1 ± 10.7, 37–80 years (mean ± SD, range). In selecting the control group, patients with a history of hypo or hyperthyroidism, thyroiditis or treated with lithium and/or amiodarone were excluded. All three groups were balanced for age and sex.
Informed consent was obtained for each patient. The thyroid hormones levels were measured on the next morning after admission using commercial radioimmunoassay kits (Atellica IM) in the laboratory of AO San Camillo-Forlanini (Rome, Italy). According to the manufacturer’s instructions, the normal reference ranges used were: 2.3–4.2 pg/ml for fT3 (cv < 9%), 0.8–1.6 ng/dl for fT4 (cv < 10%), 0.4–3.5 μIU/ml for TSH (cv < 4%), > 100 U/ml for AbTG positivity (cv < 7%) and > 100 U/ml for AbTPO positivity (cv < 7%). In accordance with the manufacturer’s standard value, low T3 syndrome was defined by a low serum fT3 level (< 2.3 pg/ml) with low or normal serum fT4 and TSH levels. Considering our patients, TSH values reported as percentiles were: 0.100 µIU/ml 25°percentile, 1.035 µIU/ml 50°percentile, 1.948 µIU/ml 75°percentile.
Descriptive statistics were used to describe the patients’ characteristics. Proportions are presented as numbers and percentages. Continuous data are reported as mean ± SD. The association between variables was tested by the Pearson chi-square test or Fisher’s Exact test, when appropriate. Mean comparison of more than two groups was made by ANOVA with Bonferroni correction for multiple comparisons. p value ≤ 0.05 was considered statistically significant. The SPSS (21.0) statistical program was used for all analysis.
Results
Considering normal values, 43% of patients (n = 10) from group A and 73.9% of patients (n = 18) from group B showed low fT3 serum levels (Chi2 p = 0.03); TSH serum levels were low both in group A (21.7%, n = 5) and in group B (78.2%, n = 18) (Chi2 p = 0.02). No alterations in fT4 levels were observed in both groups.
According to TSH quartiles, in group A one patient presented TSH < 0,1 µIU/ml, eight patients TSH between 0.1 and 1.035 µIU/ml, 10 patients TSH between 1.036 and 1.948 µIU/ml and four patients TSH > 1.948 µIU/ml. Concerning group B 14 patients presented TSH < 0,1 µIU/ml, seven patients TSH between 0.1 and 1.035 µIU/ml, one patient TSH between 1.036 and 1.948 µIU/ml and one patient TSH > 1.948 µIU/ml.
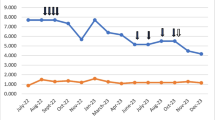
The fT3 levels were significantly lower in group A (2.40 ± 0.75 pg/ml) (mean ± SD) (p < 0.002) and group B (1.77 ± 0.68 pg/ml, p < 0.0001) compared to group C (3.14 ± 0.46 pg/ml) (Fig. 1); whereas no differences were found in fT4 levels in group A and B compared to group C (Fig. 2). Plasma TSH levels were significantly lower in group A (1.23 ± 0.89 µIU/mL, p < 0.04) and group B (0.25 ± 0.65 µIU/ml, p < 0.0001) compared to group C (1.98 ± 0.87 µIU/ml) (Fig. 3).
Between group A and B, serum fT3 levels were significantly lower in group B with respect to group A (p < 0.004) (Fig. 1) whereas no differences were found in fT4 values (Fig. 2). TSH values were significantly lower in group B with respect to group A (p < 0.02) (Fig. 3).
Discussion
In severe diseases, both acute and chronic, NTIS can occur in euthyroid patients. This condition is characterized by specific alterations in the thyroid hormonal pattern [1, 2].
The systemic homeostatic response of the organism to different noxae is complex and dynamic. In the early stages, the evident change is the reduction of fT3; with the persistence and worsening of the disease, a reduction of fT4 and TSH can progressively appear [3,4,5]. The etiopathogenesis is multifactorial and different factors, that may facilitate or provoke these alterations, have been identified, such as hypercortisolemia, increased cytokines, oxidative stress and drugs [9, 10].
COVID-19 clinical features derive from both a direct damage by SARS-Cov 2 on cells and from the immune response. The main actor involved in the acute response is interleukine-6 (IL-6). In fact, binding its membrane and soluble receptors (mIL-6R and sIL-6R), it induces chemotactic molecules secretion, such as vascular endothelial growth (VEGF), monocyte chemoattractant protein–1 (MCP-1) and CXCL8, enhancing, also, more IL-6 production [11]. The so-called “cytokine storm” describes an excessive immune response with an important secretion of inflammatory cytokines and chemokines whose plasmatic levels seem to be associated with COVID-19 severity [12]. It was observed that some mediators (IL-1β, TNF α, IL-6 and IFN-γ) can cause alterations at all levels of the pituitary-thyroid axis: central regulation, hormones production, blood transport, receptor activity, and peripheral metabolism [6, 11, 13].
NTIS appears to initially have a para-physiological, compensatory, and protective role, which reduces metabolic expenditure. Nevertheless, like other homeostatic responses of the body, it can become harmful, slowing down the healing process [5, 14].
NTIS treatment with thyroid hormones is still a topic of discussion since there is no conclusive evidence of its efficacy [2, 6, 14]. As concerns our patients, neither group A nor group B received treatment with thyroid hormones.
We found that hospitalized—COVID-19 patients presented a statistically significant reduction in fT3 and TSH values compared to euthyroid controls; furthermore, ICU patients showed lower fT3 and TSH levels.
Other studies report the finding of an NTIS picture in COVID-19 patients [15,16,17]; in particular, Gao and his group confirm that patients with severe COVID-19 show lower fT3 blood concentration [18].
It is not clear if the finding of a low fT3 and, sometimes, a reduction of TSH levels in COVID-19 patients, effectively describes a NTIS or if thyroid could be a direct target of SARS-CoV 2; the Italian group of Rotondi et al. recently demonstrated that follicular thyroid cells can express mRNA encoding for the ACE-2 receptor which is known to be SARS-Cov 2 cellular entry receptor [19]. Furthermore, Chen and his group hypothesize a specific and direct damage on the pituitary TSH-secreting cells by SARS-Cov 2 [17].
In our series, no patients with thyrotoxicosis were found, as also showed by Khoo et al. [15]; but other authors reported a prevalence of thyrotoxicosis in COVID-19 patients instead. Muller et al. focused on the hypothesis of a COVID-19 induced subacute thyroiditis. They define their observations as a combination of thyrotoxicosis and non-thyroidal illness syndrome. This study has the limitation that serum fT3 and fT4 concentrations were measured only in presence of TSH levels outside normal range [20]. Similarly, Lania and his group showed that COVID-19 patients can present thyrotoxicosis [21]. As fT3 levels and fT4 levels were only measured in patients with TSH below the normal range, it is not possible to exclude that a condition of low fT3 syndrome could be present in patients with TSH levels in the normal range.
Regarding our series, Group A data were collected immediately after patients’ hospitalization, and thus, no therapies were administrated yet. As concerns group B, during the ICU recovery, high dose of glucocorticoids were administered supporting the lowering TSH and fT3 blood levels [11]. With respect to other studies, a strength of our work is that the complete thyroid function was available for all patients.
Conclusions
During the last year few papers concerning NTIS during COVID-19 infection have been published. Our study can provide additional patients who could expand the previous series.
Patients affected by COVID-19 can present alterations of the thyroid function at different levels: on one hand NTIS and on the other hand a biochemical picture deriving from a direct damage on the thyroid (and maybe pituitary?) gland.
As expected, COVID-19 patients can present NTIS laboratory features. The progressive derangement of the clinical condition seems to be characterized by a further decrease of TSH and fT3 levels. Lower TSH values should be considered as a negative prognostic factor for these patients.
Availability of data and materials
All the authors take responsibility for the integrity of the data.
References
Pappa TA, Vagenakis AG, Alevizaki M (2011) The nonthyroidal illness syndrome in the non-critically ill patient. Eur J Clin Invest 41(2):212–220
DeGroot LJ (2000) The Non-Thyroidal Illness Syndrome. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, et al., editors. Endotext. South Dartmouth (MA)
Van den Berghe G, de Zegher F, Bouillon R (1998) Clinical review 95: acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab 83(6):1827–1834
Bello G, Pennisi MA, Montini L, Silva S, Maviglia R, Cavallaro F et al (2009) Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest 135(6):1448–1454
Wang YF, Heng JF, Yan J, Dong L (2018) Relationship between disease severity and thyroid function in Chinese patients with euthyroid sick syndrome. Medicine 97(31):11756
Van den Berghe G (2014) Non-thyroidal illness in the ICU: a syndrome with different faces. Thyroid: Off J Am Thyroid Assoc 24(10):1456–1465
Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ et al (2020) Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest 158(1):195–205
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveillance: Bull Eur sur les Maladies Transmissibles Eur Commun Dis Bull 25(3)
Abo-Zenah HA, Shoeb SA, Sabry AA, Ismail HA (2008) Relating circulating thyroid hormone concentrations to serum interleukins-6 and -10 in association with non-thyroidal illnesses including chronic renal insufficiency. BMC Endocr Disord 8:1
Qin SL, He Q, Hu L, He CY, Gao LC, Young CA et al (2018) The relationship between inflammatory factors, oxidative stress and DIO-1 concentration in patients with chronic renal failure accompanied with or without euthyroid sick syndrome. J Int Med Res 46(10):4061–4070
Croce L, Gangemi D, Ancona G, Liboa F, Bendotti G, Minelli L, et al. (2021) The cytokine storm and thyroid hormone changes in COVID-19. J Endocrinol Invest
Hu B, Huang S, Yin L (2021) The cytokine storm and COVID-19. J Med Virol 93(1):250–256
Maiden MJ, Torpy DJ (2019) Thyroid hormones in critical illness. Crit Care Clin 35(2):375–388
Lee S, Farwell AP (2016) Euthyroid sick syndrome. Compr Physiol 6(2):1071–1080
Khoo B, Tan T, Clarke SA, Mills EG, Patel B, Modi M et al (2021) Thyroid function before, during, and after COVID-19. J Clin Endocrinol Metab 106(2):e803–e811
Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY et al (2021) Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab 106(2):e926–e935
Chen M, Zhou W, Xu W (2020) Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid: Off J Am Thyroid Assoc
Gao W, Guo W, Guo Y, Shi M, Dong G, Wang G, et al. (2020) Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J Endocrinol Invest
Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. (2020) Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest
Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A et al (2020) SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 8(9):739–741
Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G (2020) Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol 183(4):381–387
Funding
This work was supported by Progetto COVID 2020 12371675 and by Ricerca Corrente on emerging and re-emerging infections both funded by Italian Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baldelli, R., Nicastri, E., Petrosillo, N. et al. Thyroid dysfunction in COVID-19 patients. J Endocrinol Invest 44, 2735–2739 (2021). https://doi.org/10.1007/s40618-021-01599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01599-0