Abstract
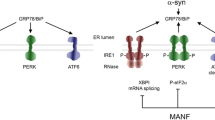
Parkinson’s disease is a neurodegenerative disorder characterised by nigrostriatal dopaminergic degeneration, and intracellular α-synuclein aggregation. Current pharmacological treatments are solely symptomatic so there is a need to identify agents that can slow or stop dopaminergic degeneration. One proposed class of therapeutics are neurotrophic factors which promote the survival of nigrostriatal dopaminergic neurons. However, neurotrophic factors need to be delivered directly to the brain. An alternative approach may be to identify pharmacological agents which can reach the brain to stimulate neurotrophic factor expression and/or their signalling pathways in dopaminergic neurons. BMP2 is a neurotrophic factor that is expressed in the human substantia nigra; exogenous BMP2 administration protects against dopaminergic degeneration in in vitro models of PD. In this study, we investigated the neurotrophic potential of two FDA-approved drugs, quinacrine and niclosamide, that are modulators of BMP2 signalling. We report that quinacrine and niclosamide, like BMP2, significantly increased neurite length, as a readout of neurotrophic action, in SH-SY5Y cells and dopaminergic neurons in primary cultures of rat ventral mesencephalon. We also show that these effects of quinacrine and niclosamide require the activation of BMP-Smad signalling. Finally, we demonstrate that quinacrine and niclosamide are neuroprotective against degeneration induced by the neurotoxins, MPP+ and 6-OHDA, and by viral-mediated overexpression of α-synuclein in vitro. Collectively, this study identifies two drugs, that are safe for use in patients' to 'are approved for human use, that exert neurotrophic effects on dopaminergic neurons through modulation of BMP-Smad signalling. This rationalises the further study of drugs that target the BMP-Smad pathway as potential neuroprotective pharmacotherapy for Parkinson’s disease.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson's disease. Lancet (London, England) 373(9680):2055–2066
Kelly MJ, O'Keeffe GW, Sullivan AM (2015) Viral vector delivery of neurotrophic factors for Parkinson's disease therapy. Expert Rev Mol Med 17:e8
Paul G, Sullivan AM (2019) Trophic factors for Parkinson's disease: where are we and where do we go from here? Eur J Neurosci 49(4):440–452
Sullivan AM, Toulouse A (2011) Neurotrophic factors for the treatment of Parkinson's disease. Cytokine Growth Factor Rev 22(3):157–165
Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H et al (1997) Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science (New York, NY) 275(5301):838–841
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L et al (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science (New York, NY) 290(5492):767–773
Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J 3rd, Gasmi M et al (2006) Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60(6):706–715
Ramaswamy S, McBride JL, Herzog CD, Brandon E, Gasmi M, Bartus RT et al (2007) Neurturin gene therapy improves motor function and prevents death of striatal neurons in a 3-nitropropionic acid rat model of Huntington's disease. Neurobiol Dis 26(2):375–384
Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, Bringas J, Pivirotto P et al (2009) Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther 20(12):1627–1640
Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN et al (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9(5):589–595
Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line—derived neurotrophic factor. J Neurosurg 102(2):216–222
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G et al (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59(3):459–466
Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS (2005) Intraputamenal infusion of glial cell line–derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 57(2):298–302
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM et al (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 54(3):403–414
Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W et al (2015) Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol 78(2):248–257
Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D et al (2019) Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson's disease. Brain. 142(3):512–525
Whone AL, Boca M, Luz M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D et al (2019) Extended Treatment with glial cell line-derived neurotrophic factor in parkinson's disease. J Parkinsons Dis 9:301–313
O'Keeffe GW, Hegarty S, Sullivan A (2017) Targeting bone morphogenetic protein signalling in midbrain dopaminergic neurons as a therapeutic approach in Parkinson's disease. Neuronal Signaling
Goulding SR, Sullivan AM, O'Keeffe GW, Collins LM (2020) The potential of bone morphogenetic protein 2 as a neurotrophic factor for Parkinson's disease. Neural Regen Res 15(8):1432–1436
Goulding SR, Sullivan AM, O'Keeffe GW, Collins LM (2019) Gene co-expression analysis of the human substantia nigra identifies BMP2 as a neurotrophic factor that can promote neurite growth in cells overexpressing wild-type or A53T α-synuclein. Parkinsonism Relat Disord 64:194–201
Ghebes CA, van Lente J, Post JN, Saris DB, Fernandes H (2017) High-throughput screening assay identifies small molecules capable of modulating the BMP-2 and TGF-beta1 Signaling pathway. SLAS Discov 22(1):40–50
Chen W, Mook RA Jr, Premont RT, Wang J (2018) Niclosamide: beyond an antihelminthic drug. Cell Signal 41:89–96
Ehsanian R, Van Waes C, Feller SM (2011) Beyond DNA binding - a review of the potential mechanisms mediating quinacrine's therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun Signal 9:13
Hegarty SV, Sullivan AM, Keeffe GW (2016) Protocol for evaluation of neurotrophic strategies in Parkinson’s disease-related dopaminergic and sympathetic neurons in vitro. 2016
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD et al (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4(1):33–41
Jordan J, Bottner M, Schluesener HJ, Unsicker K, Krieglstein K (1997) Bone morphogenetic proteins: neurotrophic roles for midbrain dopaminergic neurons and implications of astroglial cells. Eur J Neurosci 9(8):1699–1709
Reiriz J, Espejo M, Ventura F, Ambrosio S, Alberch J (1999) Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. J Neurobiol 38(2):161–170
Hegarty SV, Sullivan AM, O'Keeffe GW (2013) BMP2 and GDF5 induce neuronal differentiation through a Smad dependant pathway in a model of human midbrain dopaminergic neurons. Mol Cell Neurosci 56:263–271
Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K (1995) Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. J Neurosci Res 42(5):724–732
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER Jr et al (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 60(1):69–73
Hegarty SV, Collins LM, Gavin AM, Roche SL, Wyatt SL, Sullivan AM, O’Keeffe GW (2014) Canonical BMP-Smad signalling promotes neurite growth in rat midbrain dopaminergic neurons. NeuroMolecular Med 16(2):473–489
Weiss A, Attisano L (2013) The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2(1):47–63
Wrana JL, Attisano L (2000) The Smad pathway. Cytokine Growth Factor Rev 11(1-2):5–13
Weber D, Kotzsch A, Nickel J, Harth S, Seher A, Mueller U, Sebald W, Mueller TD (2007) A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct Biol 7:6
Mueller TD, Nickel J (2012) Promiscuity and specificity in BMP receptor activation. FEBS Lett 586(14):1846–1859
Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J et al (2014) Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis 1(1):87–105
Heinecke K, Seher A, Schmitz W, Mueller TD, Sebald W, Nickel J (2009) Receptor oligomerization and beyond: a case study in bone morphogenetic proteins. BMC Biol 7:59
Hegarty SV, Sullivan AM, O'Keeffe GW (2017) Endocytosis contributes to BMP2-induced Smad signalling and neuronal growth. Neurosci Lett 643:32–37
Tariq M, Khan HA, Al Moutaery K, Al DS (2001) Protective effect of quinacrine on striatal dopamine levels in 6-OHDA and MPTP models of Parkinsonism in rodents. Brain Res Bull 54(1):77–82
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 136(8):2419–2431
O'Keeffe GW, Sullivan AM (2018) Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson's disease. Parkinsonism Relat Disord 56:9–15 (56:9-15)
Talk AC, Muzzio IA, Matzel LD (1997) Phospholipases and arachidonic acid contribute independently to sensory transduction and associative neuronal facilitation in Hermissenda type B photoreceptors. Brain Res 751(2):196–205
Farooqui AA, Ong WY, Horrocks LA (2006) Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev 58(3):591–620
Kudo I, Matsuzawa A, Imai K, Murakami M, Inoue K (1996) Function of type II phospholipase A2 in dopamine secretion by rat neuronal PC12 cells. J Lipid Mediat Cell Signal 14(1-3):25–31
Brunner J, Gattaz WF (1995) Intracerebral injection of phospholipase A2 inhibits dopamine-mediated behavior in rats: possible implications for schizophrenia. Eur Arch Psychiatry Clin Neurosci 246(1):13–16
Klivenyi P, Beal MF, Ferrante RJ, Andreassen OA, Wermer M, Chin MR, Bonventre JV (1998) Mice deficient in group IV cytosolic phospholipase A2 are resistant to MPTP neurotoxicity. J Neurochem 71(6):2634–2637
Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schäfer-Korting M, Pfeilschifter J et al (2004) Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem 279(34):35255–35262
Kadri H, Lambourne OA, Mehellou Y (2018) Niclosamide, a drug with many (re)purposes. ChemMedChem. 13(11):1088–1091
Alasadi A, Chen M, Swapna GVT, Tao H, Guo J, Collantes J, Fadhil N, Montelione GT et al (2018) Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis 9(2):215
Kazlauskaite A, Muqit MM (2015) PINK1 and Parkin – mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson's disease. FEBS J 282(2):215–223
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S et al (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science (New York, NY) 304(5674):1158–1160
Barini E, Miccoli A, Tinarelli F, Mulholland K, Kadri H, Khanim F, Stojanovski L, Read KD et al (2018) The anthelmintic drug niclosamide and its analogues activate the parkinson's disease associated protein kinase pinK1. Chembiochem 19(5):425–429
Funding
This publication has emanated from research conducted with the financial support of a RISAM PhD scholarship from Munster Technological University (R00094948) and a research grant from Science Foundation Ireland (SFI) under the grant numbers 15/CDA/3498 (G.O’K.).
Author information
Authors and Affiliations
Contributions
SG performed the experiments, analysed the data and co-wrote the manuscript. ML, AS, LC and GOK co-wrote the manuscript. SG, LC and GOK designed the experiments. LC and GOK supervised the study.
Corresponding authors
Ethics declarations
Ethics Approval
Animal tissue was obtained under license with full ethical approval.
Consent to Participate
N/A.
Consent for Publication
N/A.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
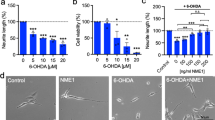
Fig. 1. SH-SY5Y cells as a tool to study drugs affecting BMP-Smad signalling. Representative photomicrographs showing immunocytochemical staining for (a) BMPR1B, (b) BMPR2 and (c) Smad1/5/8 expression in SH-SY5Y cells. (d) Representative photomicrographs of immunocytochemical staining of phospho-Smad1/5/8 and (e) intensity of phospho-Smad1/5/8 as measured using ELISA in SH-SY5Y cells after treatment with 50 ng/ml rhBMP2 with and without 1 μg/ml dorsomorphin for 2 h. (f) Total neurite length and (h) representative photomicrographs of SH-SY5Y cells after treatment with 10, 50 or 200 ng/ml rhBMP2 for 24 h. (g) Total neurite length and (i) representative photomicrographs of SH-SY5Y cells after treatment with 1 μg/ml dorsomorphin with and without 50 ng/ml rhBMP2 for 24 h. All data are presented as mean ± SEM from at least three experiments. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. control; +++p < 0.001 vs rhBMP2. One-way ANOVA with Tukey’s post-hoc test). Fig. 2. AAV-α-synuclein affects neurite length of cultured dopaminergic neurons in a concentration-dependent manner. (a) Graph and (b) representative photomicrographs of TH+ neurons transduced with AAV2/6-GFP or AAV2/6-α-synuclein at the MOI’s indicated for 5 DIV. Scale bar = 50 μm. All data are presented as mean ± SEM from at least three experiments. (**p < 0.01 vs. control; Unpaired Student’s t test). (DOCX 528 kb)
Rights and permissions
About this article
Cite this article
Goulding, S.R., Lévesque, M., Sullivan, A.M. et al. Quinacrine and Niclosamide Promote Neurite Growth in Midbrain Dopaminergic Neurons Through the Canonical BMP-Smad Pathway and Protect Against Neurotoxin and α-Synuclein-Induced Neurodegeneration. Mol Neurobiol 58, 3405–3416 (2021). https://doi.org/10.1007/s12035-021-02351-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02351-8