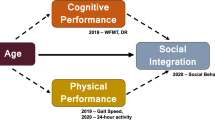
Abstract
Dual declines in gait speed and cognitive performance are associated with increased risk of developing dementia. Characterizing the patterns of such impairments therefore is paramount to distinguishing healthy from pathological aging. Nonhuman primates such as vervet/African green monkeys (Chlorocebus aethiops sabaeus) are important models of human neurocognitive aging, yet the trajectory of dual decline has not been characterized. We therefore (1) assessed whether cognitive and physical performance (i.e., gait speed) are lower in older aged animals; (2) explored the relationship between performance in a novel task of executive function (Wake Forest Maze Task—WFMT) and a well-established assessment of working memory (delayed response task—DR task); and (3) examined the association between baseline gait speed with executive function and working memory at 1-year follow-up. We found (1) physical and cognitive declines with age; (2) strong agreement between performance in the novel WFMT and DR task; and (3) that slow gait is associated with poor cognitive performance in both domains. Our results suggest that older aged vervets exhibit a coordinated suite of traits consistent with human aging and that slow gait may be a biomarker of cognitive decline. This integrative approach provides evidence that gait speed and cognitive function differ across the lifespan in female vervet monkeys, which advances them as a model that could be used to dissect relationships between trajectories of dual decline over time.




Similar content being viewed by others
Change history
12 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11357-021-00427-w
References
Statistics. National Center for Health Statistics. Health, United States, 2018. Hyattsville: Centers for Disease Control; 2019.
Olshansky SJ. From lifespan to healthspan. JAMA. 2018;320(13):1323–4. https://doi.org/10.1001/jama.2018.12621.
Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: Prospective cohort study. BMJ. 2009;339(nov10 2):b4460–b. https://doi.org/10.1136/bmj.b4460.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. https://doi.org/10.1001/jama.2010.1923.
Hoogendijk EO, Rijnhart JJM, Skoog J, Robitaille A, van den Hout A, Ferrucci L, et al. Gait speed as predictor of transition into cognitive impairment: Findings from three longitudinal studies on aging. Exp Gerontol. 2020;129:110783. https://doi.org/10.1016/j.exger.2019.110783.
Okely JA, Deary IJ. Associations between declining physical and cognitive functions in the Lothian Birth Cohort 1936. J Gerontol A Biol Sci Med Sci. 2020;75(7):1393–402. https://doi.org/10.1093/gerona/glaa023.
Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, Gardette V, Annweiler C, Beauchet O, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci. 2012;67(4):425–32. https://doi.org/10.1093/gerona/glr177.
Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–37. https://doi.org/10.1093/gerona/gls256.
Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229:89–93. https://doi.org/10.1016/j.jns.2004.11.009.
Montero-Odasso M, Speechley M, Muir-Hunter SW, Pieruccini-Faria F, Sarquis-Adamson Y, Hachinski V, et al. Dual decline in gait speed and cognition is associated with future dementia: Evidence for a phenotype. Age Ageing. 2020;49:995–1002. https://doi.org/10.1093/ageing/afaa106.
Grande G, Haaksma ML, Rizzuto D, Melis RJF, Marengoni A, Onder G, et al. Co-occurrence of cognitive impairment and physical frailty, and incidence of dementia: Systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;107:96–103. https://doi.org/10.1016/j.neubiorev.2019.09.001.
Tian Q, Resnick SM, Mielke MM, Yaffe K, Launer LJ, Jonsson PV, et al. Association of dual decline in memory and gait speed with risk for dementia among adults older than 60 years: A multicohort individual-level meta-analysis. JAMA Netw Open. 2020;3(2):e1921636. https://doi.org/10.1001/jamanetworkopen.2019.21636.
Seo EH, Kim H, Lee KH, Choo IH. Altered executive function in pre-mild cognitive impairment. J Alzheimers Dis. 2016;54(3):933–40. https://doi.org/10.3233/JAD-160052.
Kirova AM, Bays RB, Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. Biomed Res Int. 2015;2015:748212–9. https://doi.org/10.1155/2015/748212.
Chai WJ, Abd Hamid AI, Abdullah JM. Working memory from the psychological and neurosciences perspectives: A review. Front Psychol. 2018;9:401. https://doi.org/10.3389/fpsyg.2018.00401.
Bocchi A, Carrieri M, Lancia C, Quaresima V, Piccardi L. The Key of the Maze: The role of mental imagery and cognitive flexibility in navigational planning. Neurosci Lett. 2017;651:146–50. https://doi.org/10.1016/j.neulet.2017.05.009.
Wray C, Kowalski A, Mpondo F, Ochaeta L, Belleza D, DiGirolamo A, et al. Executive functions form a single construct and are associated with schooling: Evidence from three low- and middle- income countries. PLoS One. 2020;15(11):e0242936. https://doi.org/10.1371/journal.pone.0242936.
Sakamoto K, Saito N, Yoshida S, Mushiake H. Dynamic axis-tuned cells in the monkey lateral prefrontal cortex during a path-planning task. J Neurosci. 2020;40(1):203–19. https://doi.org/10.1523/JNEUROSCI.2526-18.2019.
Handing E, Rapp S, Chen SH, Rejeski WJ, Wiberg M, Bandeen-Roche K, et al. Heterogeneity in association between cognitive function and gait speed among older adults: An integrative data analysis study. J Gerontol: Ser A. 2020;25:glaa211. https://doi.org/10.1093/gerona/glaa211.
Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: The Tasmanian study of cognition and gait. J Gerontol A Biol Sci Med Sci. 2015;70(10):1226–32. https://doi.org/10.1093/gerona/glv066.
Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, Shorr R, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29(3-4):156–62. https://doi.org/10.1159/000111577.
Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, Shinkai S. A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J Gerontol A Biol Sci Med Sci. 2012;67(7):796–803. https://doi.org/10.1093/gerona/glr243.
Rasmussen LJH, Caspi A, Ambler A, Broadbent JM, Cohen HJ, d'Arbeloff T, et al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw Open. 2019;2(10):e1913123. https://doi.org/10.1001/jamanetworkopen.2019.13123.
Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19(1):186. https://doi.org/10.1186/s12877-019-1199-7.
Finkel D, Ernsth-Bravell M, Pedersen NL. Temporal dynamics of motor functioning and cognitive aging. J Gerontol A Biol Sci Med Sci. 2016;71(1):109–16. https://doi.org/10.1093/gerona/glv110.
Best JR, Liu-Ambrose T, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, et al. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci. 2016;71(12):1616–23. https://doi.org/10.1093/gerona/glw066.
Watson NL, Rosano C, Boudreau RM, Simonsick EM, Ferrucci L, Sutton-Tyrrell K, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–100. https://doi.org/10.1093/gerona/glq111.
Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol A Biol Sci Med Sci. 2016;71(10):1243–53. https://doi.org/10.1093/gerona/glv035.
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW. t Hart BA et al. Why primate models matter. Am J Primatol. 2014;76(9):801–27. https://doi.org/10.1002/ajp.22281.
Gray DT, Barnes CA. Experiments in macaque monkeys provide critical insights into age-associated changes in cognitive and sensory function. Proc Natl Acad Sci U S A. 2019;116:26247–54. https://doi.org/10.1073/pnas.1902279116.
Darusman HS, Call J, Sajuthi D, Schapiro SJ, Gjedde A, Kalliokoski O, et al. Delayed response task performance as a function of age in cynomolgus monkeys (Macaca fascicularis). Primates. 2014;55(2):259–67. https://doi.org/10.1007/s10329-013-0397-8.
Watson SL, Shively CA, Voytko ML. Can puzzle feeders be used as cognitive screening instruments? Differential performance of young and aged female monkeys on a puzzle feeder task. Am J Primatol. 1999;49:195–202.
Sadoun A, Rosito M, Fonta C, Girard P. Key periods of cognitive decline in a nonhuman primate model of cognitive aging, the common marmoset (Callithrix jacchus). Neurobiol Aging. 2019;74:1–14. https://doi.org/10.1016/j.neurobiolaging.2018.10.003.
Joly M, Ammersdorfer S, Schmidtke D, Zimmermann E. Touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLoS One. 2014;9(10):e109393. https://doi.org/10.1371/journal.pone.0109393.
Hopkins WD, Mareno MC, Webb SJN, Schapiro SJ, Raghanti MA, Sherwood CC. Age-related changes in chimpanzee (Pan troglodytes) cognition: Cross-sectional and longitudinal analyses. In: bioRxiv; 2020. https://doi.org/10.1101/2020.04.27.064626.
Huber HF, Gerow KG, Nathanielsz PW. Walking speed as an aging biomarker in baboons (Papio hamadryas). J Med Primatol. 2015;44(6):373–80. https://doi.org/10.1111/jmp.12199.
Shively CA, Willard SL, Register TC, Bennett AJ, Pierre PJ, Laudenslager ML, et al. Aging and physical mobility in group-housed Old World monkeys. Age (Dordr). 2012;34(5):1123–31. https://doi.org/10.1007/s11357-011-9350-1.
Thompson ME, Machanda ZP, Fox SA, Sabbi KH, Otali E, Thompson Gonzalez N, et al. Evaluating the impact of physical frailty during ageing in wild chimpanzees (Pan troglodytes schweinfurthii). Philos Trans R Soc Lond Ser B Biol Sci. 2020;375(1811):20190607. https://doi.org/10.1098/rstb.2019.0607.
Yamada Y, Kemnitz JW, Weindruch R, Anderson RM, Schoeller DA, Colman RJ. Caloric restriction and healthy life span: Frail phenotype of nonhuman primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J Gerontol A Biol Sci Med Sci. 2018;73(3):273–8. https://doi.org/10.1093/gerona/glx059.
Appt SE, Ethun KF. Reproductive aging and risk for chronic disease: Insights from studies of nonhuman primates. Maturitas. 2010;67(1):7–14. https://doi.org/10.1016/j.maturitas.2010.03.028.
Jasinska AJ, Schmitt CA, Service SK, Cantor RM, Dewar K, Jentsch JD, et al. Systems biology of the vervet monkey. ILAR J. 2013;54(2):122–43. https://doi.org/10.1093/ilar/ilt049.
Cox LA, Olivier M, Spradling-Reeves K, Karere GM, Comuzzie AG, VandeBerg JL. Nonhuman primates and translational research-cardiovascular disease. ILAR J. 2017;58(2):235–50. https://doi.org/10.1093/ilar/ilx025.
Latimer CS, Shively CA, Keene CD, Jorgensen MJ, Andrews RN, Register TC, et al. A nonhuman primate model of early Alzheimer's disease pathologic change: Implications for disease pathogenesis. Alzheimers Dement. 2019;15(1):93–105. https://doi.org/10.1016/j.jalz.2018.06.3057.
Neff EP. Animal models of Alzheimer’s disease embrace diversity. Lab Anim. 2019;48:255–9.
Allard M, Lèbre V, Robine JM, Calment J. Jeanne Calment: from Van Gogh's time to ours, 122 extraordinary years. New York: WH Freeman & Company; 1998.
Weigl R. Longevity of mammals in captivity; from the Living Collections of the world: A list of mammalian longevity in captivity. Stuttgart: Kleine Senckenberg-Reihe; 2005.
Justice JN, Silverstein-Metzler MG, Uberseder B, Appt SE, Clarkson TB, Register TC, et al. Relationships of depressive behavior and sertraline treatment with walking speed and activity in older female nonhuman primates. Geroscience. 2017;39(5-6):585–600. https://doi.org/10.1007/s11357-017-9999-1.
Beran MJ, Parrish AE, Futch SE, Evans TA, Perdue BM. Looking ahead? Computerized maze task performance by chimpanzees (Pan troglodytes), rhesus monkeys (Macaca mulatta), capuchin monkeys (Cebus apella), and human children (Homo sapiens). J Comp Psychol. 2015;129(2):160–73. https://doi.org/10.1037/a0038936.
Kim K, Jeon H-A, Seo J, Park J, Won J, Yeo H-G, et al. Evaluation of cognitive function in adult rhesus monkeys using the finger maze test. Appl Anim Behav Sci. 2020;224:104945. https://doi.org/10.1016/j.applanim.2020.104945.
Tsuchida J, Kawasaki K, Sankai T, Kubo N, Terao K, Koyama T, et al. New type of puzzle-task finger maze learning in Macaca fascicularis. Int J Primatol. 2003;24(2):261–70. https://doi.org/10.1023/A:1023040931101.
Jacobsen CF. Functions of frontal association areas in primates. Arch Neurol Psychiatr. 1935;33:358–69.
Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Physiol Psychol. 1971;77(2):212–20. https://doi.org/10.1037/h0031649.
James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27(52):14358–64. https://doi.org/10.1523/JNEUROSCI.4508-07.2007.
Primates M, Altschul DM, Beran MJ, Bohn M, Call J, DeTroy S, et al. Establishing an infrastructure for collaboration in primate cognition research. PLoS One. 2019;14(10):e0223675. https://doi.org/10.1371/journal.pone.0223675.
Mattison JA, Vaughan KL. An overview of nonhuman primates in aging research. Exp Gerontol. 2017;94:41–5. https://doi.org/10.1016/j.exger.2016.12.005.
Shively CA, Appt SA, Chen H, Day SM, Frye BM, Shaltout HA, et al. Mediterranean diet, stress resilience, and aging in nonhuman primates. Neurobiol Stress. 2020;13:100254. https://doi.org/10.1016/j.ynstr.2020.100254.
Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. Stress, the HPA axis, and nonhuman primate well-being: A review. Appl Anim Behav Sci. 2013;143(2-4):135–49. https://doi.org/10.1016/j.applanim.2012.10.012.
Taylor JH, Mustoe AC, Hochfelder B, French JA. Reunion behavior after social separation is associated with enhanced HPA recovery in young marmoset monkeys. Psychoneuroendocrinology. 2015;57:93–101. https://doi.org/10.1016/j.psyneuen.2015.03.019.
Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18(10):1376–85. https://doi.org/10.1038/nn.4087.
McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–16. https://doi.org/10.1016/0959-4388(95)80028-X.
Lyons DM, Lopez JM, Yang C, Schatzberg AF. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. J Neurosci. 2000;20(20):7816–21.
Snyder-Mackler N, Sanz J, Kohn JN, Voyles T, Pique-Regi R, Wilson ME, et al. Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc Natl Acad Sci U S A. 2019;116(4):1219–28. https://doi.org/10.1073/pnas.1811758115.
Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, Zuberbuhler K. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat Commun. 2016;7:13361. https://doi.org/10.1038/ncomms13361.
Guarino A, Forte G, Giovannoli J, Casagrande M. Executive functions in the elderly with mild cognitive impairment: a systematic review on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment Health. 2020;24(7):1028–45. https://doi.org/10.1080/13607863.2019.1584785.
Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M, Casagrande M. Executive functions in Alzheimer disease: A systematic review. Front Aging Neurosci. 2019;10:437. https://doi.org/10.3389/fnagi.2018.00437.
Acknowledgements
We thank Chrissy Long and Justin Herr for their advice and assistance during this study.
Funding
This work was supported by several mechanisms, including the following: National Institutes of Health (NIH) R01HL087103 (CAS), NIH RF1AG058829 (CAS & SC), P30 AG049638 (SC), Intramural Grant from the Department of Pathology, Wake Forest School of Medicine (CAS), Wake Forest Claude Pepper Older Americans Independence Center grant P30 AG21332 (SK), Vervet Research Colony (P40-OD010965) (MJ), and the Wake Forest Clinical and Translational Science Institute (NCATS UL1TR001420).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 2997 kb)
About this article
Cite this article
Frye, B.M., Valure, P.M., Craft, S. et al. Temporal emergence of age-associated changes in cognitive and physical function in vervets (Chlorocebus aethiops sabaeus). GeroScience 43, 1303–1315 (2021). https://doi.org/10.1007/s11357-021-00338-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00338-w