Abstract
Purpose
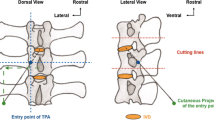
In the context of regenerative medicine strategies, based in particular on the injection of regenerative cells, biological factors, or biomaterials into the nucleus pulposus (NP), two main routes are used: the transpedicular approach (TPA) and the transannular approach (TAA). The purpose of our study was to compare the long-term consequences of the TPA and the TAA on intervertebral disc (IVD) health through a longitudinal follow-up in an ovine model.
Methods
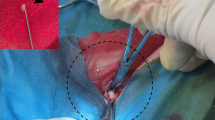
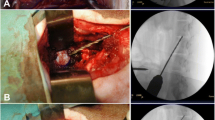
The TPA and the TAA were performed on 12 IVDs from 3 sheep. Six discs were left untreated and used as controls. The route and injection feasibility, as well as the IVD environment integrity, were assessed by MRI (T2-weighted signal intensity), micro-CT scan, and histological analyses (Boos’ scoring). The sheep were assessed at 1, 3, and 7 months.
Results
Both the TPA and the TAA allowed access to the NP. They both induced NP degeneration, as evidenced by a decrease in the T2wsi and an increase in the Boos’ scores. The TPA led to persistent end-plate defects and herniation of NP tissue (Schmorl’s node-like) after 7 months as well as the presence of osseous fragments in the NP.
Conclusions
The TPA induced more severe lesions in IVDs and vertebrae compared to the TAA. The lesions induced by the TPA are reason to consider whether or not this route is optimal for studying IVD regenerative medicine approaches.





Similar content being viewed by others
References
Luoma K, Riihimäki H, Luukkonen R et al (2000) Low back pain in relation to lumbar disc degeneration. Spine 25:487–492
Andersson GB (1999) Epidemiological features of chronic low-back pain. Lancet 354:581–585. https://doi.org/10.1016/S0140-6736(99)01312-4
Airaksinen O, Brox JI, Cedraschi C et al (2006) Chapter 4 European guidelines for the management of chronic nonspecific low back pain On behalf of the COST B13 Working Group on Guidelines for Chronic Low Back Pain. Eur Spine J 15: 192–300
Harrop JS, Youssef JA, Maltenfort M et al (2008) Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine 33:1701–1707. https://doi.org/10.1097/BRS.0b013e31817bb956
Colombier P, Camus A, Lescaudron L et al (2014) Intervertebral disc regeneration: a great challenge for tissue engineers. Trends Biotechnol 32:433–435. https://doi.org/10.1016/j.tibtech.2014.05.006
Henry N, Colombier P, Lescaudron L et al (2014) Regenerative medicine of the intervertebral disc: from pathophysiology to clinical application. Med Sci M/S 30:1091–1100. https://doi.org/10.1051/medsci/20143012012
Crevensten G, Walsh AJL, Ananthakrishnan D et al (2004) Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 32:430–434
Sakai D, Mochida J, Iwashina T et al (2006) Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 27:335–345. https://doi.org/10.1016/j.biomaterials.2005.06.038
Orozco L, Soler R, Morera C et al (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92:822–828. https://doi.org/10.1097/TP.0b013e3182298a15
Pettine K, a., Murphy MB, Suzuki RK, Sand TT, (2015) Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33:146–156. https://doi.org/10.1002/stem.1845
Alini M, Eisenstein SM, Ito K et al (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17:2–19. https://doi.org/10.1007/s00586-007-0414-y
Hunter CJ, Matyas JR, Duncan NA (2004) Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat 205:357–362. https://doi.org/10.1111/j.0021-8782.2004.00352.x
Mageed M, Berner D, Jülke H et al (2013) Is sheep lumbar spine a suitable alternative model for human spinal researches? Morphometrical comparison study. Lab Anim Res 29:183–189. https://doi.org/10.5625/lar.2013.29.4.183
Wilke HJ, Kettler A, Claes LE (1997) Are sheep spines a valid biomechanical model for human spines? Spine 22:2365–2374
Kapural L, Goyle A (2007) Imaging for provocative discography and minimally invasive percutaneous procedures for treatment of discogenic lower back pain. Tech Reg Anesth Pain Manag 11:73–80. https://doi.org/10.1053/j.trap.2007.02.013
Oehme D, Goldschlager T, Rosenfeld J et al (2012) Lateral surgical approach to lumbar intervertebral discs in an ovine model. Sci World J 2012:873726. https://doi.org/10.1100/2012/873726
Sobajima S, Kompel JF, Kim JS et al (2005) A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine 30:15–24
Michalek AJ, Funabashi KL, Iatridis JC (2010) Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J 19:2110–2116. https://doi.org/10.1007/s00586-010-1473-z
Li D, Yang H, Huang Y et al (2014) Lumbar intervertebral disc puncture under C-arm fluoroscopy: a new rat model of lumbar intervertebral disc degeneration. Exp Anim 63:227–234. https://doi.org/10.1538/expanim.63.227
Masuda K, Aota Y, Muehleman C et al (2004) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture : correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14
Wang JL, Tsai YC, Wang YH (2007) The leakage pathway and effect of needle gauge on degree of disc injury post anular puncture: a comparative study using aged human and adolescent porcine discs. Spine 32:1809–1815. https://doi.org/10.1097/BRS.0b013e31811ec282
Carragee EJ, Don AS, Hurwitz EL et al (2009) 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine 34:2338–2345. https://doi.org/10.1097/BRS.0b013e3181e234b5
Elliott DM, Yerramalli CS, Beckstein JC et al (2008) The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 33:588–596. https://doi.org/10.1097/BRS.0b013e318166e0a2
Kim KS, Yoon ST, Li J et al (2005) Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine 30:33–37
Vadalà G, Russo F, Pattappa G et al (2013) The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine 38:E319–324. https://doi.org/10.1097/BRS.0b013e318285bc4a
Vadalà G, De Strobel F, Bernardini M et al (2013) The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J 22(Suppl 6):S972–978. https://doi.org/10.1007/s00586-013-3007-y
Le Fournier L, Fusellier M, Halgand B et al (2017) The transpedicular surgical approach for the development of intervertebral disc targeting regenerative strategies in an ovine model. Eur Spine J 26:2072–2083. https://doi.org/10.1007/s00586-017-5199-z
Vinatier C, Gauthier O, Masson M et al (2008) Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J Biomed Mater Res Part A 89:176–185. https://doi.org/10.1002/jbm.a.31988
Boos N, Weissbach S, Rohrbach H et al (2002) Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27:2631–2644. https://doi.org/10.1097/01.BRS.0000035304.27153.5B
Omlor GW, Lorenz S, Nerlich AG et al (2018) Disc cell therapy with bone-marrow-derived autologous mesenchymal stromal cells in a large porcine disc degeneration model. Eur Spine J 27:2639–2649. https://doi.org/10.1007/s00586-018-5728-4
Omlor GW, Fischer J, Kleinschmitt K et al (2014) Short-term follow-up of disc cell therapy in a porcine nucleotomy model with an albumin-hyaluronan hydrogel: in vivo and in vitro results of metabolic disc cell activity and implant distribution. Eur Spine J 23:1837–1847. https://doi.org/10.1007/s00586-014-3314-y
Sheng SR, Wang XY, Xu HZ et al (2010) Anatomy of large animal spines and its comparison to the human spine: a systematic review. Eur Spine J 19:46–56. https://doi.org/10.1007/s00586-009-1192-5
Beckstein JC, Sen S, Schaer TP et al (2008) Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine 33:E166–E173. https://doi.org/10.1097/BRS.0b013e31824d911c
Omlor GW, Nerlich AG, Wilke H et al (2009) A new porcine in vivo animal model of disc degeneration: response of annulus fibrosus cells, chondrocyte-like nucleus pulposus cells, and notochordal nucleus pulposus cells to partial nucleotomy. Spine 34:2730–2739. https://doi.org/10.1097/BRS.0b013e3181b723c9
Clouet J, Vinatier C, Merceron C et al (2009) The intervertebral disc: from pathophysiology to tissue engineering. Jt Bone Spine 76:614–618. https://doi.org/10.1016/j.jbspin.2009.07.002
Reitmaier S, Schmidt H, Ihler R et al (2013) Preliminary investigations on intradiscal pressures during daily activities: an in vivo study using the merino sheep. PLoS ONE 8:1–10. https://doi.org/10.1371/journal.pone.0069610
Wognum S, Huyghe JM, Baaijens FPT (2006) Influence of osmotic pressure changes on the opening of existing cracks in 2 intervertebral disc models. Spine 31:1783–1788. https://doi.org/10.1097/01.brs.0000227267.42924.bb
Vadalà G, Sowa G, Hubert M et al (2012) Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 6:348–355. https://doi.org/10.1002/term.433
Vadalà G, Russo F, De Strobel F et al (2018) Novel stepwise model of intervertebral disc degeneration with intact annulus fibrosus to test regeneration strategies. J Orthop Res 36:1–9. https://doi.org/10.1002/jor.23905
May RD, Frauchiger DA, Albers CE et al (2018) Inhibitory effects of human primary intervertebral disc cells on human primary osteoblasts in a co-culture system. Int J Mol Sci 19:4. https://doi.org/10.3390/ijms19041195
Urban JPG, Smith S, Fairbank JCT (2004) Nutrition of the intervertebral disc: spine. Spine 29:2700–2709
Benneker LM, Heini PF, Alini M et al (2005) Vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine 30:167–173. https://doi.org/10.1097/01.brs.0000150833.93248.09
Määttä JH, MacGregor A, Karppinen J et al (2016) The relationship between Modic changes and intervertebral disc degeneration. BMC Musculoskelet Disord 17:371. https://doi.org/10.1186/s12891-016-1198-1
Haschtmann D, Stoyanov JV, Gédet P, Ferguson SJ (2008) Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J 17:289–299. https://doi.org/10.1007/s00586-007-0509-5
Cinotti G, Della Rocca C, Romeo S et al (2005) Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine 30:174–180. https://doi.org/10.1097/01.brs.0000150530.48957.76
Sun W, Zhang K, Zhao C et al (2013) Quantitative T2 mapping to characterize the process of intervertebral disc degeneration in a rabbit model. BMC Musculoskelet Disord 14:357. https://doi.org/10.1186/1471-2474-14-357
Clouet J, Fusellier M, Camus A, et al (2018) Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. pii: S0169–409X(18)30079-6
Illien-Jünger S, Pattappa G, Peroglio M et al (2012) Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine 37:1865–1873. https://doi.org/10.1097/BRS.0b013e3182544a8a
Wangler S, Peroglio M, Menzel U et al (2019) Mesenchymal stem cell homing into intervertebral discs enhances the Tie2-positive progenitor cell population, prevents cell death, and induces a proliferative response. Spine 44:1613–1622. https://doi.org/10.1097/BRS.0000000000003150
Cuellar JM, Stauff MP, Herzog RJ et al (2016) Does provocative discography cause clinically important injury to the lumbar intervertebral disc? A 10-year matched cohort study. Spine J 16:273–280. https://doi.org/10.1016/j.spinee.2015.06.051
Kim KH, Park JY, Park HS et al (2015) Which iodinated contrast media is the least cytotoxic to human disc cells? Spine J. https://doi.org/10.1016/j.spinee.2015.01.015
Karaarslan N, Yilmaz I, Ozbek H et al (2019) Are radio-contrast agents commonly used in discography toxic to the intact intervertebral disc tissue cells? Basic Clin Pharmacol Toxicol 124:181–189. https://doi.org/10.1111/bcpt.13112
Acknowledgements
This study was supported by grants from the Société Française de Neurochirurgie, the Société Française de Chirurgie du Rachis, the Agence de la Biomédecine, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Région des Pays de la Loire, ANR générique 2014 (REMEDIV project), the Fondation pour la Recherche Médicale FRM Bioingénierie (DBS20131128442), the Région des Pays de la Loire Research Program "Longévité Mobilité Autonomie" (LMA). The authors gratefully acknowledge the technical assistance that they received from the personnel of the CRIP (i.e. Patrice Roy, Christian Raphael, Stéphane Madec, Ingrid Leborgne, and Gildas Vaillant) and the SC3M platform (Service Commun de Microscopie électronique, Microcaractérisation et Morpho-histologie-imagerie fonctionnelle of the INSERM UMS016-SFR François Bonamy), as well as Sophie Domingues (Longdom publishing) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no potential conflicts of interests.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were adhered to. All procedures involving animals performed in this study were in accordance with the ‘‘3Rs’’ rule (Replacement, Reduction, and Refinement), the ethics standards of the institution and the practice at which the study was performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Decante, C., Clouet, J., Hamel, A. et al. Collateral effects of targeting the nucleus pulposus via a transpedicular or transannular surgical route: a combined X-ray, MRI, and histological long-term descriptive study in sheep. Eur Spine J 30, 585–595 (2021). https://doi.org/10.1007/s00586-020-06602-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06602-5