Abstract
Introduction
In nephropathic cystinosis (NC), adherence to cysteamine remains challenging; poor adherence is worsening the disease progression with a decline of kidney function and increase of extrarenal morbidities. Our objective was to describe adherence to cysteamine in NC patients, using electronic monitoring systems.
Methods
Patients with confirmed NC, aged > 4 years and receiving oral cysteamine (short acting or delayed release formulation as standard of care) from 3 French reference centers, were included. Adherence to treatment was primarily assessed as the percentage of days with a good adherence score, adherence score rating from 0 (poor) to 2 (good). A descriptive analysis was performed after 1-year follow-up.
Results
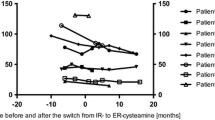
Seventeen patients (10 girls, median age: 13.9 (5.4–33.0) years) were included. Median age at diagnosis was 17.0 (3.0–76.9) months and age at start of cysteamine was 21.0 (15.5–116.3) months. Median daily dose of cysteamine was 1.05 (0.55–1.63) g/m2/day. Over the year, the median percentage of days with a good adherence score was 80 (1–99)% decreasing to 68 (1–99)% in patients > 11 years old. The median of average number of hours covered by treatment in a day was 22.5 (6.1–23.9) versus 14.9 (9.2–20.5) hours for delayed release versus short acting cysteamine.
Conclusion
Our data are the first describing a rather good adherence to cysteamine, decreasing in adolescents and adults. We described a potential interest of the delayed release formulation. Our data highlight the need for a multidisciplinary approach including therapeutic education and individualized approaches in NC patients transitioning to adulthood.
Graphical abstract



Similar content being viewed by others
Abbreviations
- ACD:
-
Acid citric citrate dextrose
- AUC:
-
Area under the curve
- BCA:
-
BiCinchoninic acid Assay
- BSA:
-
Body surface area
- CIC:
-
Clinical Investigation Center (Centre d’Investigation Clinique)
- CKD:
-
Chronic kidney disease
- CPP:
-
Comité de Protection des Personnes
- CTNS:
-
Cystinosis gene
- DR:
-
Delayed release
- GFR:
-
Glomerular filtration rate
- LC-MS/MS:
-
Liquid chromatography–tandem mass spectroscopy
- MEMs:
-
Medication event monitoring system
- NC:
-
Nephropathic cystinosis
- ORKID:
-
Orphan Kidney Disease
- SA:
-
Short acting
References
Gahl WA, Thoene JG, Schneider JA (2002) Cystinosis. N Engl J Med 347:111–121
Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C (1998) A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18:319–324
Kalatzis V, Cherqui S, Antignac C, Gasnier B (2001) Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J 20:5940–5949
Haq MR, Kalatzis V, Gubler MC, Town MM, Antignac C, Van't Hoff WG, Woolf AS (2002) Immunolocalization of cystinosin, the protein defective in cystinosis. J Am Soc Nephrol 13:2046–2051
Broyer M (2000) Cystinosis from childhood to adulthood. Nephrologie 21:13–18
Lichtenberg-Geslin L, Bacchetta J, Bertholet-Thomas A, Dubourg L, Cochat P (2015) Tubulopathies. EMC Pediatr 50:1–16
Vargas-Poussou R (2002) Molecular aspects of renal tubule diseases. Arch Pediatr 9 Suppl 2:163s–166s
Brodin-Sartorius A, Tete MJ, Niaudet P, Antignac C, Guest G, Ottolenghi C, Charbit M, Moyse D, Legendre C, Lesavre P, Cochat P, Servais A (2012) Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int 81:179–189
Thoene JG, Oshima RG, Crawhall JC, Olson DL, Schneider JA (1976) Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest 58:180–189
Pisoni RL, Thoene JG, Christensen HN (1985) Detection and characterization of carrier-mediated cationic amino acid transport in lysosomes of normal and cystinotic human fibroblasts. Role in therapeutic cystine removal? J Biol Chem 260:4791–4798
Schneider JA (2004) Treatment of cystinosis: simple in principle, difficult in practice. J Pediatr 145:436–438
Ariceta G, Lara E, Camacho JA, Oppenheimer F, Vara J, Santos F, Munoz MA, Cantarell C, Gil Calvo M, Romero R, Valenciano B, Garcia-Nieto V, Sanahuja MJ, Crespo J, Justa ML, Urisarri A, Bedoya R, Bueno A, Daza A, Bravo J, Llamas F, Jimenez Del Cerro LA (2015) Cysteamine (Cystagon(R)) adherence in patients with cystinosis in Spain: successful in children and a challenge in adolescents and adults. Nephrol Dial Transplant 30:475–480
Kleta R, Bernardini I, Ueda M, Varade WS, Phornphutkul C, Krasnewich D, Gahl WA (2004) Long-term follow-up of well-treated nephropathic cystinosis patients. J Pediatr 145:555–560
Gahl WA, Balog JZ, Kleta R (2007) Nephropathic cystinosis in adults: natural history and effects of oral cysteamine therapy. Ann Intern Med 147:242–250
Doyle M, Werner-Lin A (2015) That eagle covering me: transitioning and connected autonomy for emerging adults with cystinosis. Pediatr Nephrol 30:281–291
Bjork J, Nyman U, Berg U, Delanaye P, Dubourg L, Goffin K, Grubb A, Hansson M, Littmann K, Asling-Monemi K, Bokenkamp A, Pottel H (2019) Validation of standardized creatinine and cystatin C GFR estimating equations in a large multicentre European cohort of children. Pediatr Nephrol 34:1087–1098
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Kamoun P, Vianey-Saban C, Aupetit J, Boyer S, Chadefaux-Vekemans B (1999) Measurement of cystine in granulocytes and leucocytes: methodological aspects. In: Broyer M (ed) Cystinosis. Elsevier, Amsterdam, pp 86–92
Ged C, Jean G, Tete MJ, Broyer M, Kamoun P (1991) Intra-leukocyte cystine in cystinosis treated with cysteamine. Ann Biol Clin (Paris) 49:482–486
Piraud M, Vianey-Saban C, Bourdin C, Acquaviva-Bourdain C, Boyer S, Elfakir C, Bouchu D (2005) A new reversed-phase liquid chromatographic/tandem mass spectrometric method for analysis of underivatised amino acids: evaluation for the diagnosis and the management of inherited disorders of amino acid metabolism. Rapid Commun Mass Spectrom 19:3287–3297
Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, Alam T (2012) Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm 18:527–539
Zaugg V, Korb-Savoldelli V, Durieux P, Sabatier B (2018) Providing physicians with feedback on medication adherence for people with chronic diseases taking long-term medication. Cochrane Database Syst Rev 1:CD012042
Levtchenko EN, van Dael CM, de Graaf-Hess AC, Wilmer MJ, van den Heuvel LP, Monnens LA, Blom HJ (2006) Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol 21:110–113
Bouazza N, Treluyer JM, Ottolenghi C, Urien S, Deschenes G, Ricquier D, Niaudet P, Chadefaux-Vekemans B (2011) Population pharmacokinetics and pharmacodynamics of cysteamine in nephropathic cystinosis patients. Orphanet J Rare Dis 6:86
Besouw M, Tangerman A, Cornelissen E, Rioux P, Levtchenko E (2012) Halitosis in cystinosis patients after administration of immediate-release cysteamine bitartrate compared to delayed-release cysteamine bitartrate. Mol Genet Metab 107:234–236
Ahlenstiel-Grunow T, Kanzelmeyer NK, Froede K, Kreuzer M, Drube J, Lerch C, Pape L (2017) Switching from immediate- to extended-release cysteamine in nephropathic cystinosis patients: a retrospective real-life single-center study. Pediatr Nephrol 32:91–97
Servais A, Goizet C, Bertholet-Thomas A, Decramer S, Llanas B, Choukroun G, Novo R (2015) Cystinosis in adults: a systemic disease. Nephrol Ther 11:152–159
Acknowledgements
We wish to thank the clinical investigation centers which participated in the recruitment and follow-up of the patients: CIC of Robert Debré (Dr Florentia Kaguelidou, Mr. François Luc,,Mrs. Christine Samy, Dr. Ying Wang and Mrs. Setareh Zarrabian), CIC of Montpellier (Mr Hugues Chevassus, Mrs. Mirna Khalil and Mrs. Cécile Rouge).
We also wish to thank the team in Lyon that participated actively in the preparation and conduct of the study:
-
Valentine Bréant and Bettina Colombet, hospital pharmacists;
-
Luc Anselmini, Florine Coeurdray, Aurélie Félizard, Delphine Grosjean, Flore Maillot, and Damien Thomas, lab technicians;
-
Nathalie Touil, neuropsychologist and Aurore Curie, neuropediatrician;
-
Christelle Bonifas and Jacques Fleury, ophthalmologists;
-
Béatrice Abel, Najet Belghali, Eleonore Brocard, Catherine Caire, Corine Di-Cara, and Mélanie Romier, study nurses;
-
And a very special thanks to the clinical research associates who greatly contributed to the quality of the data: Fanny Abad, Caroline Douchet, and Hanane Gheit.
We finally thank the patients’ organizations (Cystinose France, Vaincre les Maladies Lysosomales and Association pour l’Information et la Recherche sur les maladies Rénales Génétiques) and the patients who agreed to participate in this study and gave their time for this project.
Availability of data and material
Data can be shared following regulatory and confidentiality requirements to other researchers on request after thorough review of the objective.
Funding
This project was supported by a grant from the ministry of health through the “Programme Hospitalier de Recherche Clinique National” in 2009.
Author information
Authors and Affiliations
Contributions
S Gaillard (article author, study project manager, protocol writing, methodology, interpretation of results); A Bertholet-Thomas (main project coordinator, protocol writing, patient recruitment); P Cochat (protocol writing, patient recruitment); L Roche, C Mercier, F Subtil (statistical plan and analysis); S Lemoine, G Deschênes, D Morin, B Ranchin, J Bacchetta (patient recruitment); C Vianey-Saban, C Acquaviva-Bourdain (cysteine and cysteamine assessments); Eurielle Bodénan, Valérie Laudy (Study coordination, Clinical research associates), Cécile Rouges, Setareh Zarrabian (local study coordinators); P Nony, B Kassai (methodology, interpretation of results). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consents to participate
This study was approved by the French Ethic committee (CPP Sud Est II) on 8 September 2010. The study was conducted in accordance with the French legislation, the Good Clinical Practice and the Declaration of Helsinki. Consents or assents were obtained from all participants and/or their parents/legal guardians before any procedures started.
Consent for publication
Not applicable.
Competing interests
Investigators (and their teams) of CrYSTobs study were also investigators of Raptor studies which covered the development of Procysbi®. The clinical investigation center of Lyon coordinated the Raptor studies in France.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 601 kb).
Rights and permissions
About this article
Cite this article
Gaillard, S., Roche, L., Lemoine, S. et al. Adherence to cysteamine in nephropathic cystinosis: A unique electronic monitoring experience for a better understanding. A prospective cohort study: CrYSTobs. Pediatr Nephrol 36, 581–589 (2021). https://doi.org/10.1007/s00467-020-04722-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04722-0