Abstract
Objective
To assess the contour and volumetric changes of hard and soft tissues after guided bone regeneration (GBR) using two types of barrier membranes together with a xenogeneic bone substitute in dehiscence-type defects around dental implants.
Material and methods
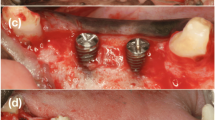
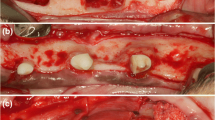
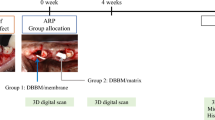
In 8 Beagle dogs, after tooth extraction, two-wall chronified bone defects were developed. Then, implants were placed with a buccal dehiscence defect that was treated with GBR using randomly: (i) deproteinized bovine bone mineral (DBBM) covered by a synthetic polylactic membrane (test group), (ii) DBBM plus a porcine natural collagen membrane (positive control) and (iii) defect only covered by the synthetic membrane (negative control group). Outcomes were evaluated at 4 and 12 weeks. Micro-CT was used to evaluate the hard tissue volumetric changes and STL files from digitized cast models were used to measure the soft tissues contour linear changes.
Results
Test and positive control groups were superior in terms of volume gain and contour changes when compared with the negative control. Soft tissue changes showed at 4 weeks statistically significant superiority for test and positive control groups compared with negative control. After 12 weeks, the results were superior for test and positive control groups but not statistically significant, although, with a lesser magnitude, the negative control group exhibited gains in both, soft and hard tissues.
Conclusions
Both types of membranes (collagen and synthetic) attained similar outcomes, in terms of hard tissue volume gain and soft tissue contours when used in combination with DBBM
Clinical relevance
Synthetic membranes were a valid alternative to the “gold standard” natural collagen membrane for treating dehiscence-type defects around dental implants when used with a xenogeneic bone substitute scaffold





Similar content being viewed by others
References
Hammerle CHF, Tarnow D (2018) The etiology of hard and soft tissue deficiencies at dental implants: a narrative review. J Clin Periodontol 45(Suppl 20):S267–S277
Araujo MG, Lindhe J (2005) Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 32:212–218
Tan WL, Wong TLT, Wong MCM, Lang NP (2012) A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res 23(Suppl. 5):1–21
Moraschini V, Poubel LC, Ferreira V, dos Sp Barboza E (2015) Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg 44:377–388
Caneva M, Salata LA, de Souza SS, Baffone G, Lang NP, Botticelli D (2010) Influence of implant positioning in extraction sockets on osseointegration: histomorphometric analyses in dogs. Clin Oral Implants Res 21:43–49
Grunder U, Gracis S, Capelli M (2005) Influence of 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent 25:113–119
Benic GI, Hämmerle CHF (2014) Horizontal bone augmentation by means of guided bone regeneration. Periodontol 66:13–40
Sanz-Sánchez I, Carrillo de Albornoz A, Figuero E, Schwarz F, Jung R, Sanz M, Thoma D (2018) Effects of lateral bone augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res 29(Suppl. 15):18–31
Sanz M, Dahlin C, Apatzidou D et al (2019) Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol 46(Suppl. 21):82–91
Haugen HJ, Lyngstadaas SP, Rossi F, Perale G (2019) Bone grafts: which is the ideal biomaterial? J Clin Periodontol 46(Suppl. 21):92–102
Sanz M, Vignoletti F (2015) Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent Mater 31:640–647
Thoma DS, Bienz SP, Figuero E, Jung RE, Sanz-Martín I (2019) Efficacy of lateral bone augmentation performed simultaneously with dental implant placement: a systematic review and meta-analysis. J Clin Periodontol 46(Suppl. 21):257–276
Dahlin C, Linde A, Gottlow J, Nyman S (1988) Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 81:672–676
Omar O, Elgali I, Dahlin C, Thomsen P (2019) Barrier membranes: more than the barrier effect? J Clin Periodontol 46(Suppl. 21):103–123
Zitzmann NU, Naef R, Scharer P (1997) Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral Maxillofac Implants 12(6):844–852
Simion M, Baldoni M, Rossi P, Zaffe D (1994) A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent 14:167–180
Schwarz F, Rothamel D, Herten M, Sager M, Becker J (2006) Angiogenesis pattern of native and cross-linked collagen membranes: an immunohistochemical study in the rat. Clin Oral Implants Res 17:403–409
Schwarz F, Rothamel D, Herten M, Sager M, Ferrari D, Becker J (2008) Immunohistochemical characterization of guided bone regeneration at dehiscence-type defect using different barrier membranes: an experimental study in dogs. Clin Oral Implants Res 19:402–415
Rothamel D, Schwarz F, Sager M, Herten M, Becker J (2005) Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res 16:369–378
Gentile P, Chiono V, Tonda-Turo C, Ferreira AM, Ciardelli G (2011) Polymeric membranes for guided bone regeneration. Biotechnol J 6(10):1187–1197
Gottlow J, Laurell L, Lundgren D, Mathisen T, Nyman S, Rylander H, Bogentoff C (1994) Periodontal tissue response to a new bioresorbable guided tissue regeneration device: a longitudinal study in monkeys. Int J Periodontics Restorative Dent 14:437–449
Lundgren D, Laurell L, Gottlow J, Rylander H, Torbjörn M, Nyman S, Rask M (1995) The influence of the design of two different bioresorbable barriers on the results of guided tissue regeneration therapy. An intra-individual comparative study in the monkey. J Periodontol 66:605–612
Matsumoto G, Hoshino J, Kinoshita Y, Sugita Y, Kubo K, Maeda H, Ikada Y, Kinoshita Y (2012) Alveolar bone regeneration using poly-(lactic acid-co-glycolic acid-co-e-caprolactone) porous membrane with collagen sponge containing basic fibroblast growth factor: an experimental study in the dog. J Biomater Appl 27(4):485–493
Won JY, Park CY, Bae JH, Ahn G, Kim C, Lim DH, Cho DW, Yun WS, Shim JH (2016) Evaluation of 3D printed PCL/PLGA/-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed Mater 11(5):055013
Sanz-Martın I, Sailer I, Hammerle CHF, Thoma DS (2016) Soft tissue stability and volumetric changes after 5 years in pontic sites with or without soft tissue grafting: a retrospective cohort study. Clin Oral Implants Res 27:969–974
Sanz-Martin I, Ferrantino L, Vignoletti F, Nunez J, Baldini N, Duvina M, Alcaraz J, Sanz M (2017) Contour changes after guided bone regeneration of large non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a porcine-derived collagen membrane: an experimental in vivo investigation. Clin Oral Investig 22(3):1273–1283
Sanz Martin I, Vignoletti F, Nuñez J, Permuy M, Muñoz F, Sanz-Esporrin J, Fierravanti L, Shapira L, Sanz M (2017) Hard and soft tissue integration of immediate and delayed implants with a modified coronal macro design: histological, micro CT and volumetric soft tissue changes from a pre-clinical in vivo study. J Clin Periodontol 44(8):842–853
Basler T, Naenni N, Schneider D, Hammerle CHF, Jung R, Thoma DS (2018) Randomized controlled clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: 3-year results. Clin Oral Implants Res 29(5):499–507
Di Raimondo R, Sanz-Esporrin J, Pla R, Sanz-Martin I, Luengo F, Vignoletti F, Nuñez J, Sanz M (2019) Alveolar crests contour changes after guided bone regeneration using different biomaterials: an experimental in vivo investigation. Clin Oral Investig. https://doi.org/10.1007/s00784-019-03092-8
Khobragade P, Jain A, Setlur Nagesh SV, Andreana S, Dziak R, Sunkara SK, Ionita CN (2015) Micro-computed tomography (CT) based assessment of dental regenerative therapy in the canine mandible model. Proc SPIE Int Soc Opt Eng 17:9417
Baek YJ, Kim JH, Song JM, Zoon SY, Kim HS, Shin SH (2016) Chitin-fibroin-hydroxyapatite membrane for guided bone regeneration: micro-computed tomography evaluation in a rat model. Maxillofac Plast Reconstr Surg 38(1):14
Vignoletti F, Abrahamsson I (2012) Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J Clin Periodontol 39(Suppl. 12):6–27
Song JM, Shin SH, Kim YD, Lee JY, Baek YJ, Yoon SY, Kim HS (2014) Comparative study of chitosan/fibroin–hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: micro-computed tomography analysis. Int J Oral Sci 6(2):87–93
Sanz M, Ferrantino L, Vignoletti F, de Sanctis M, Berglundh T (2017) Guided bone regeneration of non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a collagen membrane: an experimental in vivo investigation. Clin Oral Implants Res 28:1466–1476
Hoornaert A, d’Arros C, Heymann MF, Layrolle P (2016) Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed Mater 11(4):045012
Lundgren D, Sennerby L, Falk H, Friberg B, Nyman S (1994) The use of a new bioresorbable barrier for guided bone regeneration in connection with implant installation. Case reports. Clin Oral Implants Res 5:177–184
Thoma DS, Jung U-W, Park J-Y, Bienz SP, Husler J, Jung RE (2017) Bone augmentation at peri-implant dehiscence defects comparing a synthetic polyethylene glycol hydrogel matrix versus standard guided bone regeneration techniques. Clin Oral Implants Res 28:e76–e83
Jung UW, Cha JK, Vignoletti F, Nunez J, Sanz J, Sanz M (2017) Simultaneous lateral bone augmentation and implant placement using a particulated synthetic bone substitute around chronic peri-implant dehiscence defects in dogs. J Clin Periodontol 44:1172–1180
Bienz SP, Jung RE, Sapata VM, Hammerle CHF, Husler J, Thoma DS (2017) Volumetric changes and peri-implant health at implant sites with or without soft tissue grafting in the esthetic zone, a retrospective case-control study with a 5-year follow-up. Clin Oral Implants Res 28:1459–1465
Antunes AA, Grossi-Oliveira GA, Martins-Neto EC, Almeida AL, Salata LA (2015) Treatment of circumferential defects with osseoconductive xenografts of different porosities: a histological, histometric, resonance frequency analysis, and micro-CT study in dogs. Clin Implant Dent Relat Res 17(Suppl 1):e202–e220
Sanz-Martin I, Permuy M, Vignoletti F, Nuñez J, Muñoz F, Sanz M (2018) A novel methodological approach using superimposed micro-CT and STL images to analyze hard and soft tissue volume in immediate and delayed implants with different cervical designs. Clin Oral Implants Res 29:986–995
Acknowledgements
We wish to acknowledge the support from Prof. Fernando Muñoz at the University of Santiago – Lugo Veterinary Hospital where the prophylometric measurements were carried out under his close supervision. We also acknowledge the support of the Biomaterials Group from Universitat Politècnica de Catalunya where the specimens were processed for micro-CT analysis.
Funding
This study was partially funded through a research contract between the Sunstar Group (Switzerland) and the University Complutense of Madrid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol of the study had been previously approved by the Regional Ethics Committee for Animal Research (EXP-20170327).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
Supplementary Fig. 1
Picture of a section of Micro-Ct where an increase in the buccal contour was observed at level of implant shoulder (H=0) (yellow line), although the first mineralized tissue to implant contact was in a more apical position (purple arrow). (PNG 5640 kb)
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Di Raimondo, R., Sanz-Esporrín, J., Sanz-Martin, I. et al. Hard and soft tissue changes after guided bone regeneration using two different barrier membranes: an experimental in vivo investigation. Clin Oral Invest 25, 2213–2227 (2021). https://doi.org/10.1007/s00784-020-03537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03537-5
Keywords
- Guided bone regeneration
- Synthetic barrier membrane
- Collagen membrane
- Prophilometric and volumetric changes
- Dental implant
- Animal model