Abstract
This study evaluated the influence of infection by Plasmodium vivax on the relations between hematological and biochemical variables and the osmotic stability of the erythrocyte membrane in a Brazilian Amazon population. A total of 72 patients with P. vivax malaria were included in the study and invited to return after 14 days, post-treatment with chloroquine and primaquine, for clinical and laboratorial reevaluations. The osmotic stability of the erythrocyte membrane was analyzed by nonlinear regression of the dependency of the absorbance of hemoglobin, released with hemolysis, as a function of the salt concentration, and it was represented by the inverse of the salt concentration at the midpoint of the curve (1/H 50) and by the variation of salt concentration, which promotes lysis (dX). Bivariate and multivariate methods were used in the analysis of the results. Prior to treatment of the disease, the erythrocytes showed greater stability, probably due to the natural selection of young and also more stable erythrocytes. The bivariate analysis showed that 1/H 50 was positively correlated with red cell distribution width (RDW), urea, triglycerides, and very low-density lipoprotein (VLDL)-cholesterol, but negatively associated with albumin, HDL-cholesterol, and indirect bilirubin, while dX was negatively associated with the mean corpuscular hemoglobin concentration. These associations were confirmed by canonical correlation analysis. Stepwise multiple linear regression showed that albumin, urea, triglycerides, and VLDL-cholesterol are the variables with the highest abilities of predicting erythrocyte stability. The bivariate analysis also showed that the hematological index RDW was related to elevated levels of bilirubin and decreased levels of albumin and urea, associated with liver damage resulting from malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Between infectious and parasitic diseases that affect humans, malaria stands out as an important public health problem in many countries of Asia, Africa, and South and Central America. In Brazil, although malaria cases have decreased during the last decade, they still account for 50–60 % of cases in the American continent (World Health Organization 2012), with 99.8 % of the cases in the Amazon region, which is considered an endemic area. In 2012, the Tropical Medicine Foundation Dr. Heitor Vieira Dourado, a reference tertiary care center located in Manaus, recorded 3,182 cases of malaria, of which 95.91 % was caused by Plasmodium vivax (Ministry for Health 2012).
The malaria cycle involves an erythrocytic phase that is responsible for the clinical characteristics of the disease (Haldar et al. 2007) since the parasitism alters the deformability of erythrocytes and impairs oxygen transport (Dondorp et al. 2000). This change in deformability, which is the result of complex interactions between membrane and cytoskeleton, was associated with the lipoperoxidation of polyunsaturated fatty acids of the erythrocyte membrane and aggregation of proteins (Akanbi et al. 2010).
The parasitism by Plasmodium also affects other properties of the erythrocyte, such as stability of the membrane. In fact, the stability of erythrocyte membrane is greatly influenced by its elasticity (Starodubtseva 2011) and composition (Perk et al. 1964), as well as all the environmental conditions that influence the mechanical and rheological properties of the erythrocyte membrane (Williams 1973).
The stability of the erythrocyte membrane can be easily determined in a concentration gradient of salt (osmotic stability) (Cunha et al. 2007; de Arvelos et al. 2013; de Freitas et al. 2008, 2010; Jain 1986; Penha-Silva et al. 2007) or chaotropic solutes such as ethanol, urea, and sodium dodecyl sulfate (Cunha et al. 2007; de Arvelos et al. 2013; de Freitas et al. 2008, 2010, 2013; Fonseca et al. 2010; Jain 1986; Penha-Silva et al. 2007).
Since malaria disturbs the stability of the erythrocyte membrane, the RBC indices, and a broad set of blood biochemical parameters, this study aimed to determine the influence of parasitism by P. vivax on the relations between those variables.
Material and methods
Ethical approval
The study was previously approved by the Ethics Review Board of the Tropical Medicine Foundation Dr. Heitor Vieira Dourado (CAAE-0075.0.115.114-11). All participants signed a written consent after being informed about the objectives of the study.
Study design
The inclusion criteria comprised age above 18 years, both genders, and no pregnancy. Participants were recruited among patients with clinical complications of malaria that were treated at the Clinical Research Ward of the Tropical Medicine Foundation Dr. Heitor Vieira Dourado (TMF-HVD), a reference care center of Manaus (Amazonas, Brazil). During the years 2011 and 2012, 80 patients with confirmed microscopic diagnosis of P. vivax mono-infection were enrolled. The patients included in this study were systematically analyzed for a possible deficiency of glucose-6-phosphate dehydrogenase (G6PDH) (Brewer et al. 1960). P. vivax mono-infection was confirmed by PCR (Snounou et al. 1993) in order to rule out mixed infections with Plasmodium falciparum. Other common infectious diseases were also ruled out through specific antibody detection (leptospirosis; hepatitis A, B, and C; and HIV), blood culture (aerobic infections), and real-time PCR (serotypes 1, 2, 3, and 4 of dengue virus).
After confirmation of the diagnosis (mono-infection by P. vivax), patients were immediately treated (first day) with chloroquine (25 mg/kg over 3 days) and primaquine (0.5 mg kg−1 day−1 for 7 days). Patients were asked to return to TMF-HVD 14 days after initiation of treatment (14th day) for clinical and laboratory reevaluations.
Quantification of parasite density (PD) in thin blood films; automated blood analyses [erythrocytes (RBC), hemoglobin (Hb), hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelets (Plt), mean platelet volume (MPV)]; and serum biochemical analyses [total cholesterol (t-C); HDL-cholesterol (HDL-C); LDL-cholesterol (LDL-C); VLDL-cholesterol (VLDL-C); glucose (Glu); aspartate aminotransferase (AST); alanine aminotransferase (ALT); gamma-glutamyltransferase (GGT); total, direct, and indirect bilirubin (TB, DB, IB, respectively); human serum albumin (HSA); and urea (urea)] were systematically performed on the first and 14th day of assessment of patients.
PD was assessed by counting the number of asexual stage parasites per 500 leukocytes and expressed in number of parasites per microliter.
Blood samples
About 15 mL of venous blood was collected by intravenous puncture into evacuated tubes with and without K3EDTA, after overnight (8–12 h) fasting. Aliquots of plasma, serum, and whole blood were stored at −70 °C.
Determination of hematological and biochemical variables
Hemogram was done using an automated system of analysis (Sysmex KX-21N, Sysmex Corporation, Mundelein, IL, USA). Lipid, renal, and hepatic profiles were performed in an automated analyzer (COBAS Mira Plus™, Roche Diagnostics, Indianapolis, IN, USA) and microplate reader (DTX 800 Multimode Detector, Beckman Coulter, Fullerton, CA, USA) using commercial kits (Labtest Diagnostica Ltda.).
Osmotic stability of erythrocytes (resistance of erythrocytes to hypotonic shock)
Duplicated aliquots of 290 μL of 0–1.0 g dL−1 NaCl solutions were added to a 96-well microplate. After pre-incubation for 10 min at 37 °C, addition of 10 μL of blood to each well, the microplate was gently mixed, incubated at 37 °C for 20 min, and then centrifuged at 1,300×g for 10 min at room temperature. The erythrocyte lysis was followed by measuring the supernatants’ absorbances at 560 nm (A 560) in a microplate reader.
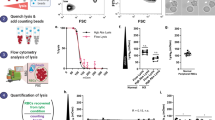
The dependence of absorbance at 560 nm (A) with the salt concentration was adjusted to a sigmoid regression line (Fig. 1), given by the Boltzmann equation.
where A 1 e A 2 are respectively the average values of A 560 at the first and second plateaus of the sigmoid (maximum and minimum), X is the NaCl concentration, H 50 is the intermediary point of the transition curve and represents the NaCl concentration necessary to promote 50 % of hemolysis, and dX is the concentration range of NaCl in the sigmoidal transition between A 1 and A 2. A 1 or A max is the maximal stationary value of absorbance and is proportional to the amount of hemoglobin released in the blood sample under the extreme hypotonic conditions of the experiment. A 2 or A min is related to the baseline hemolysis and can more appropriately represent the in vivo stability of erythrocytes since it has not been due to the in vitro lysis associated with the handling of the patient’s blood. The osmotic stability of erythrocytes was usually expressed by the values of 1/H 50 and dX since these variables are directly related to the osmotic stability of the erythrocyte membrane (Cunha et al. 2007; de Arvelos et al. 2013; de Freitas et al. 2008, 2010; Penha-Silva et al. 2007).
Sigmoidal fitting of a typical curve of hypotonic hemolysis. H 50 is the concentration of NaCl capable of promoting 50 % hemolysis. dX represents the change in NaCl concentration responsible for the full lysis of the erythrocytes population. A 1 and A 2 represent the maximum and minimum average values of absorbance at 560 nm, respectively
Statistical analyses of experimental data
The study used bivariate and multivariate statistic analyses to search for the existence of relations between the erythrocyte stability parameters and the hematological and biochemical variables. Sample size was estimated to achieve an 80 % power and 5 % significance.
Data were tested for normality using the Kolmogorov–Smirnov test. Some parameters were normally distributed, but others showed non-normal distribution. Comparison of experimental data between the two periods of this study (first and 14th days) was done using Student’s t or Mann–Whitney–Wilcoxon test, with p < 0.05 showing statistically significant difference. The existence of bivariate linear correlations between the stability parameters (1/H 50 and dX) and the hematological and biochemical variables also used the significance level of 0.05.
The two different periods of the study (first and 14th days) were also analyzed by comparing the intersection and slope of the regression lines obtained for the dependence of 1/H 50 with the different hematological and biochemical variables. This comparison was done using Student’s t test, with p < 0.05 indicating the existence of a statistically significant difference.
The multivariate analyses were performed with canonical correlations (CC) and stepwise multiple linear regression (stepwise MLR). CC is a multivariate statistical technique which can be understood as a logical extension of the MLR. Just as the MLR correlates a set of independent variables with a single dependent variable, CC correlates sets of multiple dependent variables and multiple independent variables. In situations where the researcher pursues a correlation of groups of variables, the CC technique is the most appropriate and powerful multivariate (Hair et al. 2009).
In the MLR analyses, 1/H 50 and dX were considered the dependent variables and the hematological and biochemical parameters constituted the groups of independent variables. In this kind of analysis, the partial regression coefficient measures the strength of the relation between the dependent variables (1/H 50 and dX) and a single independent variable of the group (hematological or biochemical), while the determination coefficient (R 2) measures the proportion of variance of the dependent variable (around its mean) that is explained by the whole set of independent variables. The basis of stepwise MLR is the construction of a linear combination that maximizes the correlation between a dependent variable and a set of independent variables by assigning weights to each of the dependent variables (Hair et al. 2009).
The a priori calculation of n for this analysis considered a power of 0.80 and a magnitude of the effect of 0.15, with p < 0.05 indicating statistical significance. All analyses were done with the help of BioEstat 5.0 (Mamirauá™, Belém, PA, Brazil), Origin 9.0 (MicroCal, Northampton, MA, USA), and GENES (Federal University of Viçosa, Viçosa, MG, Brazil).
Results and discussion
After ruling out deficiency of G6PDH, mixed plasmodial infections, and other comorbidities, a total of 72 patients with P. vivax malaria were included in the study (first day), but only 35 of these patients returned after 14 days for clinical and laboratorial reevaluations (14th day).
Table 1 presents the baseline characteristics (first day) of patients (n = 72) with P. vivax malaria that constituted the population of this study.
Table 2 shows the descriptive statistics of the stability parameters and hematological and biochemical variables before (first day) and after (14th day) the treatment of malaria. Although there has been a normal distribution of most variables, some variables presented a non-normal distribution.
Regarding the hematological variables, between the two study periods, there were statistical differences in Plt count, MPV, and MCH. The median Plt value was 86 × 103/μL before treatment (first day) and increased to 272 × 103/μL after the treatment (14th day; Table 2). Thrombocytopenia (Plt < 150.000/μL) is a classic feature of malaria (Alecrim 2000; Ghosh and Shetty 2008; Lacerda et al. 2011), including in the mono-infection with P. vivax (Coelho et al. 2013; Kochar et al. 2010, 2012; Tanwar et al. 2012).
The statistically significant increase observed in MCH between the first and the 14th day should be related to the tendency to normalization of hematological changes promoted by parasitism, despite patients with malaria by P. vivax or P. falciparum having impaired erythropoiesis (Srichaikul et al. 1969; Wickramasinghe and Abdalla 2000) for at least 2 weeks after treatment (Wickramasinghe et al. 1989), probably due to an increase in the phagocytic activity of macrophages in the bone marrow and/or release of cytotoxic compounds (Wickramasinghe and Abdalla 2000).
Regarding the biochemical variables, between the two study periods, there were statistically significant differences for the biochemical variables t-C, VLDL-C, LDL-C, HDL-C, tB, dB, iB, urea, and HSA (Table 2).
Transient changes in the plasma levels of cholesterol and triglycerides have been observed by many authors in different acute infections (Faucher et al. 2002; Gallin et al. 1969). Hypertriglyceridemia, hypocholesterolemia, and reduced LDL-C and HDL-C were observed in uncomplicated and complicated malaria (Gallin et al. 1969; Gavino et al. 1981; Krishna et al. 2009; Nilsson-Ehle and Nilsson-Ehle 1990; Parola et al. 2004). The same pattern of lipid abnormalities was also observed on the first day of this study (Table 2), with the values of TG, t-C, LDL-C, and HDL-C significantly different from those observed on the 14th day.
As commonly occurs in malaria, on the first day of this study, the serum levels of total, indirect, and direct bilirubin were high (Table 2). The occurrence of hyperbilirubinemia is usually explained by exacerbation of hemolysis, when indirect bilirubin predominates, or hepatic cholestasis, when direct bilirubin predominates. Regarding the erythrocyte stability parameters (1/H 50 and dX), there was a statistically significant decrease in 1/H 50 after treatment.
As the erythrocyte membrane stability was associated with a different set of hematological and biochemical variables, parasitism by Plasmodium seemed an interesting model for better understanding the set of variables that influence the stability of the erythrocyte membrane. The existence of a larger variance in many of the parameters (Table 2) favors the correlation analyses.
Table 3 shows the matrix of Spearman’s correlations between the stability parameters and hematological and biochemical variables. Within the limits of statistical significance considered in this study (p < 0.05), the variable of stability, 1/H 50, was positively correlated with the values of peripheral parasitemia, dX, RDW, urea, and VLDL-C, but negatively associated with the values of HSA, IB, HDL-C, and A min. The stability variable dX was negatively correlated with A min and MCHC.
Thus, significant direct correlations between 1/H 50 or dX and parasite density observed on the first day of the study mean that the stability of erythrocytes increases with increased parasite density (Table 3). But it decreased after treatment (14th day) since there was a significant fall in 1/H 50 compared to the first day of the study (Table 2).
The origin of this increase in membrane stability must be related to the broad set of changes that parasitism causes not only in erythrocytes but also in the patient’s bloodstream. Certainly, some of these changes should help decrease the stability of the membrane, while others must have an opposite effect. Stabilization shall be the net result of the antagonistic contributions of different factors.
These factors should comprehend the biochemical and hematological variables that showed significant correlation with the stability parameters 1/H 50 and dX (Table 3). It is important to note, however, that the existence of a bivariate correlation does not necessarily mean a cause–effect relationship, and even when a cause–effect relationship is present, it cannot predict which of the factors is a cause and which is an effect.
The stability parameter 1/H 50 was positively correlated with RDW, urea, TG, and VLDL-C, but negatively associated with HSA, HDL-C, and IB, while the stability parameter dX was negatively associated with MCHC (Table 3). These associations appear to constitute relations of cause and effect since there were significant differences in the values of all these hematological and biochemical variables between the two study periods (first and 14th days; Table 2).
Further evidence of the existence of causal relations between RBC stability and those variables comes from the comparison of the linear regression lines of the stability parameter 1/H 50 in relation to some of the studied variables (those for which the results showed normal distribution and the regression lines were statistically significant) between the two different periods of the study (first and 14th days; Table 4). The lines of 1/H 50 in relation to dX, HAS, and VLDL-C had intercept and slope coefficients which were different after treatment (14th day) as compared to before treatment (first day). The lines of 1/H 50 in relation to the variables RDW, urea, HDL-C, and IB had equal slope coefficients and different intercepts between the two periods of the study.
In order to deepen the understanding of the existing interrelations, CC analyses were performed between the groups of dependent variables (dX and 1/H 50) and groups of independent variables (Table 5) that have had significant correlations with 1/H 50 (group 1) and dX (group 2). Both groups of independent variables presented significant correlations with the first canonical pair (Tables 6 and 7). In all canonical correlations, the stability parameters have had canonical loadings (CL) that were well differentiated, depending on the relationship with the set of variables in the group.
The variables in group 1 showed significant correlations with the parameter 1/H 50 (p = 0.0115). The stability parameters showed high values of canonical loadings, but the contribution of 1/H 50 has more impact on the correlation between its group (dependent variable) and the independent variables in group 1, with a canonical loading of 0.9952 (Table 6). The variables PD, RDW, urea, and VLDL-C showed positive relationships with the first canonical pair. On the other hand, the variables HSA, HDL-C, and IB were negatively correlated with 1/H 50 and dX. The independent variable that had the greatest impact on the canonical correlation was HSA (CL = −0.6119), followed by PD (CL = 0.5534) and blood levels of VLDL-C (CL = 0.4723), always with the preservation of the directions found in the bivariate correlations between each of the stability variables and each of these independent variables (Table 3).
Regarding the variables of group 2 (Table 7), the major influences came from the hematological index MCHC and the stability parameter A min, which showed significant negative correlations with the first canonical pair.
Although the CC analysis can deal with metric and non-metric variables, it allows only detecting the existence of associations between groups of variables, but not the establishment of relations of cause and effect, which would be possible by path analysis.
Stepwise MLR allows verifying the contribution of the independent variables in explaining the variance of the dependent variable (1/H 50 and dX). The results of this analysis (Table 8) were consistent with those of the CC analyses and showed that the variables with the highest abilities of predicting RBC stability in patients with P. vivax malaria were HSA (R 2 = 12.05 %), urea (R 2 = 6.26 %), VLDL-C (R 2 = 4.95 %), and IB (R 2 = 2.64 %), in this sequence. The set of independent variables in this group accounted for approximately 27 % of the predictive ability of 1/H 50.
HSA binds cations (such as Na+ and Ca2+), free fatty acids, IB, hormones, and many drugs (Peters 1996) and possibly plays a key role as an antioxidant (Bruschi et al. 2013). The decreased levels of HSA in infected patients (first day; Table 2) can be explained by inflammation associated with the initial installation of the parasite in the liver, where that protein is produced (Attwood 2011; Ogbodo et al. 2010). Since albumin increases the osmotic stability of erythrocytes (Katchalsky et al. 1960; Williams 1973), the negative relations observed between 1/H 50 and HSA in the bivariate (Table 3), canonical correlations (Table 6), and stepwise MLR (Table 8) analyses may seem paradoxical. The explanation for this paradox may be in the fact that the decrease in HSA is a consequence of parasitism and that the increased stability occurs through another independent type of mechanism.
This other type of mechanism must include the selection of cells that are more stable at the expense of those less stable due to the proper propagation of parasite from infected to uninfected cells. This process involves the association of infected and uninfected erythrocytes by means of various serum proteins like albumin itself, fibrinogen, IgG, and IgM, with the formation of structures called rosettes (Dondorp et al. 2000; Luginbuhl et al. 2007). When infected by the parasite, young erythrocytes (reticulocytes) are preserved, while the older ones are lysed. As young erythrocytes are osmotically more resistant (Marks and Johnson 1958), this would explain the positive correlation observed between the stability of the erythrocyte (dX and 1/H 50) and parasite density.
In addition to this mechanism, based on the selection, other mechanisms of stabilization of erythrocytes may be associated with parasitism, once that the preservation of P. vivax depends on the preservation of the parasitized cell. It is important to note that this increase in erythrocyte stability does not mean a physiological benefit to the infected individual, but only to P. vivax.
Indeed, infection by P. falciparum was associated with structural changes in the membrane of the host cell, such as reduced amount of net negative charges and increased resistance to lysis induced by unsaturated fatty acids (Sabolovic et al. 1994). Furthermore, the accessibility and distribution of intra-erythrocytic antigens of Plasmodium-infected erythrocytes are different from those of the uninfected cells (Wiser et al. 1993).
Digital analysis of changes in erythrocytes infected with P. vivax showed significant changes in shape descriptors such as area, perimeter, and form factor, with increased parasitemia (Edison et al. 2011), which must change their deformability and flow properties (Manjunatha and Singh 2000). The membrane of the parasitized erythrocyte also presents electron-dense protrusions (Aikawa 1988; Bracho et al. 2006; Haldar et al. 2001) and an increase in viscosity that favors adhesion to endothelial cells of small vessels in various organs and cells, not only in infections caused by P. falciparum (Winograd et al. 2001) but as well as in those caused by P. vivax (Carvalho et al. 2010; Costa et al. 2011, 2012; Marin-Menendez et al. 2013).
1/H 50 presented a significant and positive correlation with urea. Although urea is a well-known chaotrope and promotes protein unfolding and exposure of hydrophobic groups in water (Fonseca et al. 2006; Patel et al. 2010a; Timasheff 1993), it also stabilizes the lipid bilayer of the biological membranes (Feng et al. 2002; Koynova et al. 1997), particularly against osmotic stress (Costa-Balogh et al. 2006).
1/H 50 also presented a significant and positive correlation with TG and VLDL-C (Table 3). Since the levels of TG showed a significant negative correlation with the levels of HSA, it is possible that the positive relationship between 1/H 50 and TG is associated with the decreased lipolysis of VLDL-TG by lipoprotein lipase. The existence of a strong and significant correlation between 1/H 50 and VLDL-C was also shown in the CC analysis and stepwise MLR. As the erythrocyte membrane is an important target for cholesterol from non-high-density lipoproteins (Cooper 1977), the increase in the stability of the erythrocyte membrane should also have been influenced by its cholesterol content.
The erythrocyte infected by P. vivax undergoes not only biochemical but also morphological changes that affect its physicochemical properties and functions.
RDW was the hematological variable which stood out in the correlation analyses with 1/H 50, with a pattern of positive correlation in both the bivariate (Table 3) and in CC analyses (Table 6). In fact, the osmotic stability of erythrocytes is strongly associated with RDW (Bernardino Neto 2011; Bernardino Neto et al. 2013). The RDW, which represents the percentage change in the mean corpuscular volume of the erythrocytes, was shown to be elevated in patients with malaria (Bunyaratvej et al. 1993; Koltas et al. 2007; Lathia and Joshi 2004). Although the evaluation of RDW does not have the ability of diagnosing malaria in relation to other non-malarial febrile illnesses, it is possible that RDW has, in malaria, the predictive ability that was reported for this variable in the risk of death from cardiovascular diseases (Nishizaki et al. 2012; Patel et al. 2010b; Tziakas et al. 2012) and diabetes (Malandrino et al. 2012).
RDW was also suggested as a potential prognostic index for liver disease since it was positively correlated with serum bilirubin and negatively correlated with serum albumin (Hu et al. 2013). In the present study, RDW was also positively correlated with total and direct bilirubin and negatively correlated with human serum albumin and urea (Table 3). Indeed, the elevation in direct bilirubin observed on the first day (Table 2), along with the significant positive correlation observed between direct bilirubin and γ-glutamyl transferase (Table 3), is consistent with hepatic cholestasis and liver damage.
MCHC was another hematological variable that was associated with erythrocyte stability. The stability parameter dX showed a significant negative association with MCHC in the bivariate analysis (Table 3) and also in the CC analysis, where MCHC was the second variable that contributed the most in the correlation (Table 7). Such a contribution of MCHC is consistent because an increase in MCHC values also increases the difference in the concentration gradient between the internal and external media of the cell, which causes increased water absorption and reduced stability.
The variable A min, which is also a parameter of stability, showed a significant negative association with dX in the bivariate and multivariate analysis. A min was the variable with the highest canonical loading among the variables in group 2 (Table 7) and the variable that contributed the most to dX in the stepwise MLR.
In summary, there is an increase in the stability of the erythrocyte membrane in patients suffering from malaria, which probably involves not only natural selection of young and more stable erythrocytes but also the factors that determine the values of the hematological indices MCHC and RDW, as well as the serum levels of triglycerides, VLDL-C, and urea. Moreover, the hematological index RDW was shown to be related to the elevated levels of bilirubin and decreased levels of albumin and urea, associated with liver damage resulting from malaria.
References
Aikawa M (1988) Morphological changes in erythrocytes induced by malarial parasites. Biol Cell 64(2):173–181
Akanbi OM, Odaibo AB, Ademowo OG (2010) Effect of antimalarial drugs and malaria infection on oxidative stress in pregnant women. Afr J Reprod Health 14(3):209–212
Alecrim MGC (2000) Clinical aspects, resistance and parasitary polymorphism of Plasmodium vivax malaria in Manaus. Brasília University
Attwood D (2011) Malaria in South Sudan 2: clinical features and diagnosis. Southern Sudan Med J 4(1):10–12
Bernardino Neto M (2011) Analysis of correlations between stability of erythrocyte membrane, serum lipids and hematologic variables. Federal University of Uberlandia
Bernardino Neto M et al (2013) Bivariate and multivariate analyses of the correlations between stability of the erythrocyte membrane, serum lipids and hematological variables. Biorheology. doi:10.3233/BIR-130641
Bracho C et al (2006) Caveolins and flotillin-2 are present in the blood stages of Plasmodium vivax. Parasitol Res 99(2):153–159. doi:10.1007/s00436-006-0139-6
Brewer GJ, Tarlov AR, Alving AS (1960) Methaemoglobin reduction test: a new, simple, in vitro test for identifying primaquine-sensitivity. Bull World Health Organ 22:633–640
Bruschi M, Candiano G, Santucci L, Ghiggeri GM (2013) Oxidized albumin. The long way of a protein of uncertain function. Biochim Biophys Acta 1830:5473–5479. doi:10.1016/j.bbagen.2013.04.017
Bunyaratvej A, Butthep P, Bunyaratvej P (1993) Cytometric analysis of blood cells from malaria-infected patients and in vitro infected blood. Cytometry 14(1):81–85. doi:10.1002/cyto.990140114
Carvalho BO et al (2010) On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis 202(4):638–647. doi:10.1086/654815
Coelho HC et al (2013) Thrombocytopenia in Plasmodium vivax malaria is related to platelets phagocytosis. PLoS One 8(5):e63410. doi:10.1371/journal.pone.0063410
Cooper RA (1977) Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med 297(7):371–377. doi:10.1056/NEJM197708182970707
Costa FT et al (2011) On cytoadhesion of Plasmodium vivax: raison d’etre? Mem Inst Oswaldo Cruz 106(Suppl 1):79–84
Costa FT et al (2012) On the pathogenesis of Plasmodium vivax malaria: perspectives from the Brazilian field. Int J Parasitol 42(12):1099–1105. doi:10.1016/j.ijpara.2012.08.007
Costa-Balogh FO, Wennerstrom H, Wadso L, Sparr E (2006) How small polar molecules protect membrane systems against osmotic stress: the urea–water–phospholipid system. J Phys Chem B 110(47):23845–23852. doi:10.1021/jp0632440
Cunha CC, Arvelos LR, Costa JO, Penha-Silva N (2007) Effects of glycerol on the thermal dependence of the stability of human erythrocytes. J Bioenerg Biomembr 39(4):341–347. doi:10.1007/s10863-007-9092-z
de Arvelos LR et al (2013) Bivariate and multivariate analyses of the influence of blood variables of patients submitted to Roux-en-Y gastric bypass on the stability of erythrocyte membrane against the chaotropic action of ethanol. J Membr Biol 246(3):231–242. doi:10.1007/s00232-013-9524-0
de Freitas MV et al (2008) Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol In Vitro 22(1):219–224. doi:10.1016/j.tiv.2007.07.010
de Freitas MV, de Oliveira MR, dos Santos DF, de Cassia Mascarenhas Netto R, Fenelon SB, Penha-Silva N (2010) Influence of the use of statin on the stability of erythrocyte membranes in multiple sclerosis. J Membr Biol 233(1–3):127–134. doi:10.1007/s00232-010-9232-y
de Freitas MV et al (2013) Influence of age on the concentration of hematological and biochemical variables with the stability of erythrocyte membrane in relation to sodium dodecyl sulfate. Hematology. doi:10.1179/1607845413Y.0000000145
Dondorp AM, Kager PA, Vreeken J, White NJ (2000) Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol Today 16(6):228–232
Edison M, Jeeva JB, Singh M (2011) Digital analysis of changes by Plasmodium vivax malaria in erythrocytes. Indian J Exp Biol 49(1):11–15
Faucher JF, Ngou-Milama E, Missinou MA, Ngomo R, Kombila M, Kremsner PG (2002) The impact of malaria on common lipid parameters. Parasitol Res 88(12):1040–1043. doi:10.1007/s00436-002-0712-6
Feng Y, Yu ZW, Quinn PJ (2002) Effect of urea, dimethylurea, and tetramethylurea on the phase behavior of dioleoylphosphatidylethanolamine. Chem Phys Lipids 114(2):149–157
Fonseca LC, Correa NCR, Garrote MD, da Cunha CC, Penha-Silva N (2006) Effects of the solvent composition on the stability of proteins in aqueous solutions. Quim Nova 29(3):543–548
Fonseca LC, Arvelos LR, Netto RC, Lins AB, Garrote-Filho MS, Penha-Silva N (2010) Influence of the albumin concentration and temperature on the lysis of human erythrocytes by sodium dodecyl sulfate. J Bioenerg Biomembr 42(5):413–418. doi:10.1007/s10863-010-9310-y
Gallin JI, Kaye D, O’Leary WM (1969) Serum lipids in infection. N Engl J Med 281(20):1081–1086. doi:10.1056/NEJM196911132812001
Gavino VC, Miller JS, Ikharebha SO, Milo GE, Cornwall DG (1981) Effects of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res 22:763–769
Ghosh K, Shetty S (2008) Blood coagulation in falciparum malaria—a review. Parasitol Res 102(4):571–576. doi:10.1007/s00436-007-0832-0
Hair JFJ, Black WC, Babin BJ, Anderson RE (2009) Multivariate data analysis, 7th edn. Prentice Hall, Englewood Cliffs
Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL (2001) Transport mechanisms in Plasmodium-infected erythrocytes: lipid rafts and a tubovesicular network. Int J Parasitol 31(12):1393–1401
Haldar K, Murphy SC, Milner DA, Taylor TE (2007) Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol 2:217–249. doi:10.1146/annurev.pathol.2.010506.091913
Hu Z et al (2013) Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chem Lab Med 51:1403–1408. doi:10.1515/cclm-2012-0704
Jain NC (1986) Hematology techniques. In: Jain NC (ed) Shalm’s veterinary hematology. Lea & Febiger, Philadelphia
Katchalsky A, Kedem O, Klibansky C, Devries A (1960) Flow properties of blood and other biological systems. Pergamon, Elmsford
Kochar DK et al (2010) Thrombocytopenia in Plasmodium falciparum, Plasmodium vivax and mixed infection malaria: a study from Bikaner (Northwestern India). Platelets 21(8):623–627. doi:10.3109/09537104.2010.505308
Kochar DK et al (2012) Platelet count and parasite density: independent variable in Plasmodium vivax malaria. J Vector Borne Dis 49(3):191–192
Koltas IS, Demirhindi H, Hazar S, Ozcan K (2007) Supportive presumptive diagnosis of Plasmodium vivax malaria. Thrombocytopenia and red cell distribution width. Saudi Med J 28(4):535–539
Koynova R, Brankov J, Tenchov B (1997) Modulation of lipid phase behavior by kosmotropic and chaotropic solutes: experiment and thermodynamic theory. Eur Biophys J 25(4):261–274. doi:10.1007/s002490050038
Krishna AP, Chandrika, Kumar S, Acharya M, Patil SL (2009) Variation in common lipid parameters in malaria infected patients. Indian J Physiol Pharmacol 53(3):271–274
Lacerda MV, Mourao MP, Coelho HC, Santos JB (2011) Thrombocytopenia in malaria: who cares? Mem Inst Oswaldo Cruz 106(Suppl 1):52–63
Lathia TB, Joshi R (2004) Can hematological parameters discriminate malaria from nonmalarious acute febrile illness in the tropics? Indian J Med Sci 58(6):239–244
Luginbuhl A, Nikolic M, Beck HP, Wahlgren M, Lutz HU (2007) Complement factor D, albumin, and immunoglobulin G anti-band 3 protein antibodies mimic serum in promoting rosetting of malaria-infected red blood cells. Infect Immun 75(4):1771–1777. doi:10.1128/IAI.01514-06
Malandrino N, Wu WC, Taveira TH, Whitlatch HB, Smith RJ (2012) Association between red blood cell distribution width and macrovascular and microvascular complications in diabetes. Diabetologia 55(1):226–235. doi:10.1007/s00125-011-2331-1
Manjunatha M, Singh M (2000) Digital analysis of induced erythrocyte shape changes in hypercholesterolemia under in vitro conditions. Curr Sci 79:1588–1591
Marin-Menendez A et al (2013) Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Negl Trop Dis 7(4):e2155. doi:10.1371/journal.pntd.0002155
Marks PA, Johnson AB (1958) Relationship between the age of human erythrocytes and their osmotic resistance: a basis for separating young and old erythrocytes. J Clin Invest 37(11):1542–1548. doi:10.1172/JCI103746
Ministry for Health B (2012) Epidemiological surveillance information system for malaria. http://portalweb04.saude.gov.br/sivep_malaria/default.asp Accessed June 2012
Nilsson-Ehle I, Nilsson-Ehle P (1990) Changes in plasma lipoproteins in acute malaria. J Intern Med 227(3):151–155
Nishizaki Y et al (2012) Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern Med 51(17):2271–2276
Ogbodo SO, Okeke A, Obu HA, Shu EN, Chukwurah E (2010) Nutritional status of parasitemic children from malaria endemic rural communities in eastern Nigeria. Curr Pediatr Res 14(2):131–135
Parola P, Gazin P, Patella F, Badiaga S, Delmont J, Brouqui P (2004) Hypertriglyceridemia as an indicator of the severity of falciparum malaria in returned travelers: a clinical retrospective study. Parasitol Res 92(6):464–466. doi:10.1007/s00436-003-1012-5
Patel H, Raval G, Nazari M, Heerklotz H (2010a) Effects of glycerol and urea on micellization, membrane partitioning and solubilization by a non-ionic surfactant. Biophys Chem 150(1–3):119–128. doi:10.1016/j.bpc.2010.03.015
Patel KV et al (2010b) Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 65(3):258–265. doi:10.1093/gerona/glp163
Penha-Silva N et al (2007) Influence of age on the stability of human erythrocyte membranes. Mech Ageing Dev 128(7–8):444–449. doi:10.1016/j.mad.2007.06.007
Perk K, Frei YF, Herz A (1964) Osmotic fragility of red blood cells of young and mature domestic and laboratory animals. Am J Vet Res 25:1241–1248
Peters TJ (1996) All about albumin: biochemistry, genetics, and medical applications. Academic, New York
Sabolovic D, Bouanga JC, Danis M, Mazier D, Gentilini M (1994) Alterations of uninfected red blood cells in malaria. Parasitol Res 80(1):70–73
Snounou G et al (1993) High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61:315–320
Srichaikul T, Wasanasomsithi M, Poshyachinda V, Panikbutr N, Rabieb T (1969) Ferrokinetic studies and erythropoiesis in malaria. Arch Intern Med 124(5):623–628
Starodubtseva MN (2011) Mechanical properties of cells and ageing. Ageing Res Rev 10(1):16–25. doi:10.1016/j.arr.2009.10.005
Tanwar GS et al (2012) Thrombocytopenia in childhood malaria with special reference to P. vivax monoinfection: a study from Bikaner (northwestern India). Platelets 23(3):211–216. doi:10.3109/09537104.2011.607520
Timasheff SN (1993) The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct 22:67–97. doi:10.1146/annurev.bb.22.060193.000435
Tziakas D, Chalikias G, Grapsa A, Gioka T, Tentes I, Konstantinides S (2012) Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc 51(4):243–254. doi:10.3233/CH-2012-1530
Wickramasinghe SN, Abdalla SH (2000) Blood and bone marrow changes in malaria. Bailliere Best Pract Res Clin Haematol 13(2):277–299. doi:10.1053/beha.1999.0072
Wickramasinghe SN, Looareesuwan S, Nagachinta B, White NJ (1989) Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria. Br J Haematol 72(1):91–99
Williams AR (1973) The effect of bovine and human serum albumins on the mechanical properties on human erythrocyte membranes. Biochim Biophys Acta 307(1):58–64
Winograd E, Robles WM, Caldas ML, Cortes GT (2001) Cytoadherence of the malaria-infected erythrocyte membrane to C32 melanoma cells after merozoites are released from parasitized infected cells. Parasitol Res 87(4):264–268
Wiser MF, Faur LV, Lanners HN, Kelly M, Wilson RB (1993) Accessibility and distribution of intraerythrocytic antigens of Plasmodium-infected erythrocytes following mild glutaraldehyde fixation and detergent extraction. Parasitol Res 79(7):579–586
World Health Organization (2012) World malaria report 2012. World Health Organization, Geneva
Acknowledgments
We would like to thank FAPEMIG (CDS-APQ-01862-09, CDS-APQ-02025-10, and PPM-00485-12), CAPES (PE-PNPD AUX 2718/2011), and CNPq (307705/2012-9) for the financial supports that enable the development of this study and also to Tropical Medicine Foundation Dr. Heitor Vieira Dourado, where the participants of the study were recruited.
Conflict of interest
None of the authors have financial or non-financial competing interests, as well as any other kind of interest conflict, in the work presented in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mascarenhas Netto, R.d.C., Fabbri, C., de Freitas, M.V. et al. Influence of Plasmodium vivax malaria on the relations between the osmotic stability of human erythrocyte membrane and hematological and biochemical variables. Parasitol Res 113, 863–874 (2014). https://doi.org/10.1007/s00436-013-3717-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3717-4