Abstract
Purpose
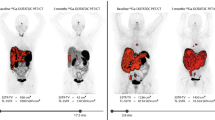
Paired imaging/therapy with radiolabeled somatostatin receptor (SSTR) antagonists is a novel approach in neuroendocrine tumors (NETs). The aim of this study was to compare tumor uptake of 68Ga-DOTA-JR11 and 177Lu-satoreotide tetraxetan (177Lu-DOTA-JR11) in patients with NETs.
Methods
As part of a prospective clinical trial, 20 patients with metastatic NETs underwent 68Ga-DOTA-JR11 PET/CT and serial imaging with 177Lu-satoreotide tetraxetan. PET/CT and SPECT/CT parameters for lesion uptake and absorbed dose of 177Lu-satoreotide tetraxetan in lesions were compared using linear regression analysis and Pearson correlation.
Results
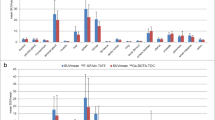
A total of 95 lesions were analyzed on 68Ga-DOTA-JR11 PET/CT and 177Lu-satoreotide tetraxetan SPECT/CT. SUVs and tumor-to-normal-tissue ratios on PET/CT and SPECT/CT were significantly correlated (p < 0.01), but the degree of correlation was modest with Pearson correlation coefficients ranging from 0.3 to 0.7. Variation in intrapatient lesional correlation was observed. Nevertheless, in all patients, the lesion SUVpeak uptake ratio for 177Lu-satoreotide tetraxetan vs. 68Ga-DOTA-JR11 was high; even in those with low uptake on 68Ga-DOTA-JR11 PET/CT (SUVpeak ≤ 10), a ratio of 8.0 ± 5.2 was noted. Correlation of SUVpeak of 68Ga-DOTA-JR11 with projected 177Lu-satoreotide tetratexan-absorbed dose (n = 42) was modest (r = 0.5, p < 0.01), while excellent correlation of SUVpeak of 177Lu-satoreotide tetraxetan with projected 177Lu-satoreotide tetraxetan-absorbed dose was noted (r = 0.9, p < 0.0001).
Conclusion
Our study shows that 68Ga-DOTA-JR11 PET can be used for patient selection and PRRT and that low tumor uptake on PET should not preclude patients from treatment with 177Lu-satoreotide tetraxetan. The ability to use single time-point SPECT/CT for absorbed dose calculations could facilitate dosimetry regimens, save costs, and improve patient convenience.





Similar content being viewed by others
References
Bodei L, Ambrosini V, Herrmann K, Modlin I. Current concepts in (68)Ga-DOTATATE imaging of neuroendocrine neoplasms: interpretation, biodistribution, dosimetry, and molecular strategies. J Nucl Med. 2017;58:1718–26. https://doi.org/10.2967/jnumed.116.186361.
Barrio M, Czernin J, Fanti S, Ambrosini V, Binse I, Du L, et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. 2017;58:756–61. https://doi.org/10.2967/jnumed.116.185587.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18.
Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med. 2010;51:875–82. https://doi.org/10.2967/jnumed.109.066134.
Velikyan I, Sundin A, Sorensen J, Lubberink M, Sandstrom M, Garske-Roman U, et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55:204–10. https://doi.org/10.2967/jnumed.113.126177.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Prasad V, et al. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–68. https://doi.org/10.1007/s00259-011-1846-5.
Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. https://doi.org/10.1007/s00259-008-0944-5.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26:1439–47.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–23. https://doi.org/10.1200/jco.2010.33.7873.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. https://doi.org/10.1200/jco.2007.15.2553.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35. https://doi.org/10.1007/s00259-011-1902-1.
Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43:518–25. https://doi.org/10.1097/mpa.0000000000000113.
Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, et al. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–41. https://doi.org/10.1073/pnas.0607761103.
Fani M, Braun F, Waser B, Beetschen K, Cescato R, Erchegyi J, et al. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J Nucl Med. 2012;53:1481–9. https://doi.org/10.2967/jnumed.112.102764.
Fani M, Del Pozzo L, Abiraj K, Mansi R, Tamma ML, Cescato R, et al. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011;52:1110–8. https://doi.org/10.2967/jnumed.111.087999.
Wild D, Fani M, Behe M, Brink I, Rivier JE, Reubi JC, et al. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J Nucl Med. 2011;52:1412–7. https://doi.org/10.2967/jnumed.111.088922.
Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55:1248–52. https://doi.org/10.2967/jnumed.114.138834.
Cescato R, Waser B, Fani M, Reubi JC. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med. 2011;52:1886–90. https://doi.org/10.2967/jnumed.111.095778.
Mansi R, Fani M. Design and development of the theranostic pair (177) Lu-OPS201/(68) Ga-OPS202 for targeting somatostatin receptor expressing tumors. J Label Compd Radiopharm. 2019;62:635–45. https://doi.org/10.1002/jlcr.3755.
Krebs S, Pandit-Taskar N, Reidy D, Beattie BJ, Lyashchenko SK, Lewis JS, et al. Biodistribution and radiation dose estimates for (68)Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:677–85. https://doi.org/10.1007/s00259-018-4193-y.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging. 2003;30:207–16. https://doi.org/10.1007/s00259-002-1023-y.
Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46. https://doi.org/10.1007/s00259-009-1072-6.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Reidy-Lagunes D, Pandit-Taskar N, O'Donoghue JA, Krebs S, Staton KD, Lyashchenko SK, et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist (177)Lu-Satoreotide Tetraxetan. Clin Cancer Res. 2019;25:6939–47. https://doi.org/10.1158/1078-0432.Ccr-19-1026.
Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, et al. 86Y-DOTA0)-D-Phe1-Tyr3-octreotide (SMT487)—a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging 2003;30:510–518. https://doi.org/10.1007/s00259-003-1117-1.
Sainz-Esteban A, Prasad V, Schuchardt C, Zachert C, Carril JM, Baum RP. Comparison of sequential planar 177Lu-DOTA-TATE dosimetry scans with 68Ga-DOTA-TATE PET/CT images in patients with metastasized neuroendocrine tumours undergoing peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2012;39:501–11. https://doi.org/10.1007/s00259-011-2003-x.
Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J, et al. Sensitivity comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase II imaging study. J Nucl Med. 2018;59:915–21. https://doi.org/10.2967/jnumed.117.199760.
Heppeler A, Froidevaux S, Maecke HR, Jermann E, Behe M, Powell P, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J. 1999;5:1974–81. https://doi.org/10.1002/(SICI)1521-3765(19990702)5:7<1974::AID-CHEM1974>3.0.CO;2-X.
Ezziddin S, Lohmar J, Yong-Hing CJ, Sabet A, Ahmadzadehfar H, Kukuk G, et al. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin Nucl Med. 2012;37:e141–7. https://doi.org/10.1097/RLU.0b013e31823926e5.
Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. 2015;17:313–8. https://doi.org/10.1007/s11307-014-0795-3.
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–9. https://doi.org/10.2967/jnumed.109.066662.
Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56:1843–8. https://doi.org/10.2967/jnumed.115.162719.
Toriihara A, Baratto L, Nobashi T, Park S, Hatami N, Davidzon G, et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:2244–51. https://doi.org/10.1007/s00259-019-04455-9.
Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–73. https://doi.org/10.1677/erc-09-0078.
Cremonesi M, Botta F, Di Dia A, Ferrari M, Bodei L, De Cicco C, et al. Dosimetry for treatment with radiolabelled somatostatin analogues. A review. The Quarterly Journal of Nuclear Medicine and Molecular Imaging : Official Publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So. 2010;54:37–51.
Acknowledgments
We gratefully acknowledge Rashid Ghani and members of the Nuclear Medicine Pharmacy; nuclear medicine nurses Ann Longing and Louise Harris for their help in patient management; clinical research coordinators Alicia Lashley, Hanh Pham, and Martha Ziolkowska, and Clinical Research Manager Bolorsukh Gansukh for their excellent support with patient flow and protocol management; the radiation safety officers and nuclear medicine technologists for their excellent technical assistance; and members of the Department of Medicine at MSK for patient referral. We also thank Leah Bassity for her assistance in editing this manuscript.
Availability of data and materials
Not applicable.
Funding
This study was supported in part by the Geoffrey Beene Cancer Research Center at MSK, and the MSK Radiochemistry and Molecular Imaging Probe Core was funded in part through NIH/NCI Cancer Center Support Grant P30 CA008748. We gratefully acknowledge funding from the Neuroendocrine Tumor Research Foundation (NETRF) (previously known as the Caring for Carcinoid Foundation). S.K. was supported in part by NIH/NCI Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746 and by the Clinical and Translational Science Center at Weill Cornell Medical Center and MSK (grant number UL1TR00457). J.S.L. acknowledges support from NIH R35 CA232130. The precursor used in this study was provided by Ipsen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. A. O’Donoghue is a consultant for Janssen Pharmaceuticals, Inc. D. Reidy is on advisory boards for Novartis, Ipsen and AAA and has received research support from Novartis, Merck, and Ipsen. J. Lewis serves on advisory boards and has received compensation (or stock) from pHLIP, Inc., Clarity Pharmaceuticals, Varian Medical Systems, InVicro, Inc., Evergreen Theragnostics, Inc., Telix Pharmaceuticals Ltd., and Trace-Ability, Inc. He is a consultant for TPG Capital, L.P., and has received research support (financial and/or reagents) from Eli Lilly and Company, Sapience Therapeutics, Inc., Mabvax Therapeutics Holdings Inc., SibTech, Inc., Thermo Fisher Scientific, ImaginAb, Inc. U.S., Merck & Company, Inc., AbbVie Inc., Bristol-Myers Squibb Company, Genentech, Inc., Y-mAbs Therapeutics, Inc., and Regeneron Pharmaceuticals, Inc. L. Bodei is a consultant (unpaid) for AAA, Ipsen, Clovis, and Curium and receives research support from AAA. W. Weber is on advisory boards and receives compensation from Bayer, Blue Earth Diagnostics, Endocyte, Pentixapharm, and ITG. He has received research support from BMS, Imaginab, Ipsen, and Piramal. N. Pandit-Taskar is a consultant, receives honoraria or serves on the advisory board for Actinium Pharma, Progenics, Medimmune/Astrazeneca, and conducted research supported by Imaginab, Genentech, Janssen. S. Krebs, E. Biegel, B.J. Beattie, and S.K. Lyashchenko declare that they have no conflicts of interest.
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Endocrinology
Rights and permissions
About this article
Cite this article
Krebs, S., O’Donoghue, J.A., Biegel, E. et al. Comparison of 68Ga-DOTA-JR11 PET/CT with dosimetric 177Lu-satoreotide tetraxetan (177Lu-DOTA-JR11) SPECT/CT in patients with metastatic neuroendocrine tumors undergoing peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 47, 3047–3057 (2020). https://doi.org/10.1007/s00259-020-04832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04832-9