Abstract
Background
Hypoparathyroidism is characterized by the absence or inadequately low circulating concentrations of the parathyroid hormone, resulting in hypocalcemia, hyperphosphatemia, and elevated fractional excretion of calcium in the urine. The use of activated vitamin D analogs and calcium supplements represent conventional therapy. Subcutaneous recombinant human parathormone [rhPTH(1–34)] has been proposed as a substitutive treatment, even to avoid side effects of vitamin D and calcium.
Objective
To assess the long-term safety and efficacy of rhPTH(1–34) in a pediatric cohort of patients with genetic hypoparathyroidism.
Methods
The study is a 9.2-year self-controlled study of six pediatric patients (four males and two females, aged 9.4 ± 5.2) with DiGeorge, hypoparathyroidism-deafness-renal dysplasia (HDR) or autoimmune-candidiasis-polyendocrinopathy-ectodermal-dysplasia (APECED) syndrome, associated with autoimmune intestinal malabsorption in a patient. The presence of clinical signs of hypocalcemia and biochemical parameters, such as calcium, phosphate, alkaline phosphatase in the blood and calcium–creatinine ratio in urine, were compared during conventional treatment and rhPTH(1–34) (teriparatide, 12.5 μg twice daily).
Results
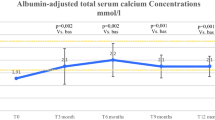
The rhPTH(1–34) treatment allowed a reduction, although not always a complete suspension, of calcium supplementation and a slight reduction of calcitriol therapy. The number of tetanic episodes was reduced in four patients during the rhPTH(1–34) treatment. Mean blood calcium, alkaline phosphatase, and phosphate did not significantly change, while a significant reduction of the urinary calcium-to-creatinine ratio (0.55 ± 0.32 vs 0.16 ± 0.09, p = 0.03) was obtained. Renal ultrasound examination showed a worsening in three patients, while it did not change in the remaining three subjects during the follow-up.
Conclusions
In children with syndromic hypoparathyroidism presented here, replacement therapy with rhPTH(1–34) allowed to maintain adequate levels of the calcium and phosphate in the blood, normalize urinary calcium excretion, and reduce tetanic episodes. In patients with low compliance to conventional therapy or intestinal malabsorption, the use of rhPTH(1–34) could be considered, also to reduce the side effects of treatment with vitamin D and calcium.


Similar content being viewed by others
References
M. Mannstadt, J.P. Bilezikian, R.V. Thakker, F.M. Hannan, B.L. Clarke, L. Rejnmark, D.M. Mitchell, T.J. Vokes, K.K. Winer, D.M. Shoback, Hypoparathyroidism. Nat. Rev. Dis. Prim. 3, 17055 (2017). https://doi.org/10.1038/nrdp.2017.55
M.L. Brandi, J.P. Bilezikian, D. Shoback, R. Bouillon, B.L. Clarke, R.V. Thakker, A.A. Khan, J.T. Potts Jr., Management of hypoparathyroidism: summary statement and guidelines. J. Clin. Endocrinol. Metab. 101(6), 2273–2283 (2016)
J. Bollerslev, L. Rejnmark, C. Marcocci, D.M. Shoback, A. Sitges-Serra, W. van Biesen, O.M. Dekkers; European Society of Endocrinology, European Society of Endocrinology Clinical Guideline: treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 173(2), G1–20 (2015)
C. Cipriani, D. Irani, J.P. Bilezikian, Safety of osteoanabolic therapy: a decade of experience. J. Bone Miner. Res. 27(12), 2419–2428 (2012)
D.L. Kendler, F. Marin, C.A.F. Zerbini, L.A. Russo, S.L. Greenspan, V. Zikan, A. Bagur, J. Malouf-Sierra, P. Lakatos, A. Fahrleitner-Pammer, E. Lespessailles, S. Minisola, J.J. Body, P. Geusens, R. Möricke, P. Lopèz-Romero, Effects of teriparatide and risedronate on new fractures in post menopausal women with severe osteroporosis (VERO): a multicenter, double-blind, double-dummy, randomized controlled trial. Lancet. 391(10117), 230–240 (2018)
N. Napoli, B.L. Langdahl, Ö. Ljunggren, E. Lespessailles, G. Kapetanos, T. Kocjan, T. Nikolic, P. Eiken, H. Petto, T. Moll, E. Lindh, F. Marin, Effects of teriparatide in patients with osteoporosis in clinical practice: 42-month results during and after discontinuation of treatment from the European Extended Forsteo® Observational Study (ExFOS). Calcif. Tissue Int. 103(4), 359–371 (2018)
B.L. Langdahl, S. Silverman, S. Fujiwara, K. Saag, N. Napoli, S. Soen, H. Enomoto, T.E. Melby, D.P. Disch, F. Marin, J.H. Krege, Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone. 116, 58–66 (2018)
S. Silverman, B.L. Langdahl, S. Fujiwara, K. Saag, N. Napoli, S. Soen, H. Enomoto, T.E. Melby, D.P. Disch, F. Marin, J.H. Krege, Reduction of hip and other fractures in patients receiving teriparatide in real-world clinical practice: integrated analysis of four prospective observational studies. Calcif. Tissue Int. 104(2), 193–200 (2019)
Food and Drug Administration (FDA). Drug Approval Package- Netpara. (2015). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125511Orig1s000TOC.cfm
European Medicine Agency (EMA). Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 20-23 February 2017. (2017). https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-20-23-february-2017
K.K. Winer, J.A. Yanovski, B. Sarani, G.B. Cutler Jr., A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1-34 in treatment of hypoparathyroidism. J. Clin. Endocrinol. Metab. 83(10), 3480–3486 (1998)
K.K. Winer, C.W. Ko, J.C. Reynolds, K. Dowdy, M. Keil, D. Peterson, L.H. Gerber, C. McGarvey, G.B. Cutler Jr., Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1-34) versus calcitriol and calcium. J. Clin. Endocrinol. Metab. 88(9), 4214–4220 (2003)
K.K. Winer, B. Zhang, J.A. Shrader, D. Peterson, M. Smith, P.S. Albert, G.B. Cutler Jr., Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J. Clin. Endocrinol. Metab. 97(2), 391–399 (2012)
K.K. Winer, J.A. Yanovski, G.B. Cutler Jr., Synthetic human parathyroid hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA 276(8), 631–636 (1996)
R.I. Gafni, L.C. Guthrie, M.H. Kelly, B.A. Brillante, C.M. Christie, J.C. Reynolds, N.A. Yovetich, R. James, M.T. Collins, Transient increased calcium and calcitriol requirements after discontinuation of human synthetic parathyroid hormone 1-34 (hPTH 1-34) replacement therapy in hypoparathyroidism. J. Bone Miner. Res. 30(11), 2112–2118 (2015)
G. Díaz-Soto, M. Mora-Porta, J. Nicolau, V. Perea, I. Halperin, M. Puig-Domingo, Efficacy and safety of long term treatment of unresponsive hypoparathyroidism using multipulse subcutaneous infusion of teriparatide. Horm. Metab. Res. 44(9), 708–710 (2012)
M. Shiohara, R. Shiozawa, K. Kurata, H. Matsuura, F. Arai, T. Yasuda, K. Koike, Effect of parathyroid hormone administration in a patient with severe hypoparathyroidism caused by gain-of-function mutation of calcium-sensing receptor. Endocr. J. 53(6), 797–802 (2006)
K.K. Winer, K.A. Fulton, P.S. Albert, G.B. Cutler Jr., Effects of pump versus twice-daily injection delivery of synthetic parathyroid hormone 1-34 in children with severe congenital hypoparathyroidism. J. Pediatr. 165(3), 556–563.e1 (2014)
K.K. Winer, N. Sinaii, J. Reynolds, D. Peterson, K. Dowdy, G.B. Cutler Jr., Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J. Clin. Endocrinol. Metab. 95(6), 2680–2688 (2010)
V. Saraff, A. Rothenbuhler, W. Högler, A. Linglart, Continuous subcutaneous recombinant parathyroid hormone (1-34) infusion in the management of childhood hypoparathyroidism associated with malabsorption. Horm. Res. Paediatr. 89(4), 271–277 (2016)
K.K. Winer, A. Kelly, A. Johns, B. Zhang, K. Dowdy, L. Kim, J.C. Reynolds, P.S. Albert, G.B. Cutler Jr., Long-term parathyroid hormone 1-34 replacement therapy in children with hypoparathyroidism. J. Pediatr. 203, 391–399.e1 (2018)
P. Matarazzo, G. Tuli, L. Fiore, A. Mussa, F. Feyles, V. Peiretti, R. Lala et al. Teriparatide (rhPTH) treatment in children with syndromic hypoparathyroidism. J. Pediatr. Endocrinol. Metab. 27(1–2), 53–59 (2014)
K.K. Winer, N. Sinaii, D. Peterson, B. Sainz Jr, G.B. Cutler Jr., Effects of once versus twice-daily parathyroid hormone 1-34 therapy in children with hypoparathyroidism. J. Clin. Endocrinol. Metab. 93(9), 3389–3395 (2008)
A. Linglart, A. Rothenbuhler, I. Gueorgieva, P. Lucchini, C. Silve, P. Bougnères, Long-term results of continuous subcutaneous recombinant PTH (1-34) infusion in children with refractory hypoparathyroidism. J. Clin. Endocrinol. Metab. 96(11), 3308–3312 (2011)
S. Sanda, K.P. Schlingmann, R.S. Newfield, Autosomal dominant hypoparathyroidism with severe hypomagnesemia and hypocalcemia, successfully treated with recombinant PTH and continuous subcutaneous magnesium infusion. J. Pediatr. Endocrinol. Metab. 21(4), 385–391 (2008)
W. Stögmann, E. Bohrn, W. Woloszczuk, Initial experiences with substitution treatment of hypoparathyroidism with synthetic human parathyroid hormone. Monatsschr. Kinderheilkd. 138(3), 141–146 (1990)
R.S. Newfield, Recombinant PTH for initial management of neonatal hypocalcemia. N. Engl. J. Med. 356(16), 1687–1688 (2007)
Y.H. Cho, M. Tchan, B. Roy, R. Halliday, M. Wilson, S. Dutt, S. Siew, C. Munns, N. Howard, Recombinant parathyroid hormone therapy for severe neonatal hypoparathyroidism. J. Pediatr. 160(2), 345–348 (2012)
G. Marcucci, G. Della Pepa, M.L. Brandi, Natpara for the treatment of hypoparathyroidism. Expert. Opin. Biol. Ther. 16(11), 1417–1424 (2016)
G. Marcucci, M.L.A. Brandi, New era for chronic management of hypoparathyroidism: parathyroid hormone peptides. Front. Horm. Res. 51, 165–171 (2019). https://doi.org/10.1159/000491047
J.L. Vahle, G.G. Long, G. Sandusky, M. Westmore, Y.L. Ma, M. Sato, Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 32(4), 426–438 (2004)
M. Sato, J. Vahle, A. Schmidt, M. Westmore, S. Smith, E. Rowley, L.Y. Ma, Abnormal bone architecture and biomechanical properties with near-lifetime treatment of rats with PTH. Endocrinology. 143(9), 3230–3242 (2002)
J.L. Vahle, M. Sato, G.G. Long, J.K. Young, P.C. Francis, J.A. Engelhardt, M.S. Westmore, Y. Linda, J.B. Nold, Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol. Pathol. 30(3), 312–321 (2002)
J. Jolette, C.E. Wilker, S.Y. Smith, N. Doyle, J.F. Hardisty, A.J. Metcalfe, T.B. Marriott, J. Fox, D.S. Wells, Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rats. Toxicol. Pathol. 34(7), 929–940 (2006)
A.H. Tashjian Jr., B.A. Chabner, Commentary on clinical safety of recombinant human parathyroid hormone 1-34 in the treatment of osteoporosis in men and postmenopausal women. J. Bone Miner. Res. 17(7), 1151–1161 (2002)
J.L. Vahle, U. Zuehlke, A. Schmidt, M. Westmore, P. Chen, M. Sato, Lack of bone neoplasms and persistence of bone efficacy in cynomolgus macaques after long-term treatment with teriparatide [rhPTH(1-34)]. J. Bone Miner. Res. 23(12), 2033–2039 (2008)
S.J. Silverberg, E. Shane, T.P. Jacobs, E. Siris, J.P. Bilezikian, A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N. Engl. J. Med. 341(17), 1249–1255 (1999)
E.B. Andrews, A.W. Gilsenan, K. Midkiff, B. Sherrill, Y. Wu, B.H. Mann, D. Masica, The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J. Bone Miner. Res. 27(12), 2429–2437 (2012)
Author contributions
All authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results (G.T., R.B. and L.D.S. contributed to the study design, the manuscript writing and final revisions; D.T., S.E., P.M. contributed to the study design and the conceptual part of this manuscript)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all parents of the individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tuli, G., Buganza, R., Tessaris, D. et al. Teriparatide (rhPTH 1–34) treatment in the pediatric age: long-term efficacy and safety data in a cohort with genetic hypoparathyroidism. Endocrine 67, 457–465 (2020). https://doi.org/10.1007/s12020-019-02128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02128-z