Abstract
Background
A high reactivation of multiple sclerosis (MS) was reported in patients treated with alemtuzumab after fingolimod. We aimed to understand whether this shift enhanced the risk for reactivation in a real-life cohort.
Methods
Subjects with relapsing MS, shifting from fingolimod to alemtuzumab were enrolled. We collected the following data: age, sex, disease duration, relapses after fingolimod withdrawal, new T2/gadolinium (Gd)-enhancing lesions in the last magnetic resonance imaging (MRI) during fingolimod and in the first, while on alemtuzumab, lymphocyte counts at alemtuzumab start, and Expanded Disability Status Scale (EDSS) before and after alemtuzumab.
Results
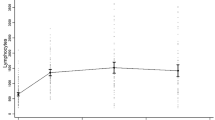
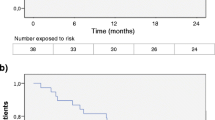
We enrolled 77 patients (women 61 (79%); mean age 36.2 years (SD 9.6), and disease duration 12.3 years (SD 6.8) at fingolimod discontinuation; median washout 1.8 months). The annualised relapse rate was 0.89 during fingolimod, 1.32 during washout, and 0.15 after alemtuzumab (p = 0.001). The EDSS changed from a median of 3 (IQR 2–4) at the end of fingolimod to 2.5 after alemtuzumab (IQR 1.5–4) (p = 0.013). The washout length and the lymphocyte count before alemtuzumab were not associated with EDSS change after alemtuzumab (p = 0.59 and p = 0.33, respectively). MRI activity decreased after alemtuzumab compared to that during fingolimod (p = 0.001). At alemtuzumab start, lymphocyte counts were < 0.8 × 103/mL in 21 patients.
Conclusions
In our cohort, alemtuzumab reduced relapse, new T2/Gd-enhancing lesions, and EDSS score, as compared to the previous periods (fingolimod/washout). These results were not related to washout length or lymphocyte counts. Therefore, a rapid initiation of alemtuzumab after fingolimod does not seem to be a risk factor for MS reactivation.



Similar content being viewed by others
References
Thompson AJ, Baranzini SE, Geurts J et al (2018) Multiple sclerosis. Lancet 391(10130):1622–1636
Lunde HMB, Assmus J, Myhr KM et al (2017) Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry 88(8):621–625
Cree BA, Hartung HP (2016) Steering through complexity: management approaches in multiple sclerosis. Curr Opin Neurol 29(3):263–271
Gafson A, Craner MJ, Matthews PM (2017) Personalised medicine for multiple sclerosis care. Mult Scler 23(3):362–369
Mandala S, Hajdu R, Bergstrom J et al (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296(5566):346–349
Cohen JA, Barkhof F, Comi G et al (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362(5):402–415
Kappos L, Radue EW, O'Connor P et al (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362(5):387–401
Calabresi PA, Radue EW, Goodin D et al (2014) Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13(6):545–556
Havla JB, Pellkofer HL, Meinl I et al (2012) Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol 69(2):262–264
Członkowska A, Smoliński Ł, Litwin T (2017) Severe disease exacerbations in patients with multiple sclerosis after discontinuing fingolimod. Neurol Neurochir Pol 51(2):156–162
Vermersch P, Radue EW, Putzki N et al (2017) A comparison of multiple sclerosis disease activity after discontinuation of fingolimod and placebo. Mult Scler J Exp Transl Clin 3(3):2055217317730096
Frau J, Sormani MP, Signori A et al (2018) Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur J Neurol 25(10):1270–1275
Hatcher SE, Waubant E, Nourbakhsh B et al (2016) Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 73(7):790–794
Cohen JA, Coles AJ, Arnold DL et al (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing–remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828
Coles AJ, Twyman CL, Arnold DL et al (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839
Haghikia A, Dendrou CA, Schneider R et al (2017) Severe B-cell-mediated CNS disease secondary to alemtuzumab therapy. Lancet Neurol 16(2):104–106
Willis M, Pearson O, Illes Z et al (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4(2):e320
Bernard-Valnet R, Pignolet B, Biotti D et al (2018) Unexpected high multiple sclerosis activity after switching from fingolimod to alemtuzumab. Mult Scler Relat Disord 25:216–218
Wehrum T, Beume LA, Stich O et al (2018) Activation of disease during therapy with alemtuzumab in 3 patients with multiple sclerosis. Neurology 90(7):e601–e605
Huhn K, Bayas A, Doerck S et al (2018) Alemtuzumab as rescue therapy in a cohort of 50 relapsing–remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol 265(7):1521–1527
McDonald WI, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50(1):121–127
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 58(6):840–846
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Wiendl H, Calabresi PA, Meuth SG (2018) Defining response profiles after alemtuzumab: rare paradoxical disease exacerbation. Neurology 90(7):309–311
Gross CC, Ahmetspahic D, Ruck T et al (2016) Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 3(6):e289
Prosperini L, Annovazzi P, Boffa L et al (2018) No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol 265(12):2851–2860
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Frau J: serves on scientific advisory boards for Biogen and Genzyme, and has received honoraria as a speaker from Merck Serono, Genzyme, Biogen and Teva. Saccà F: received personal compensations for participating in advisory boards and public speaking, or travel grants from Almirall, Biogen Idec, Forward Pharma, Merk Serono, Novartis, Pomona, Roche, Sanofi Genzyme, and Teva. Signori A: has nothing to disclose. Baroncini D: received travel grants from Genzyme, Merck and Biogen for participation in national and international congresses; he received speaking honoraria from Sanofi and Novartis, and personal compensation from Almirall for scientific publication. Fenu G: has received honoraria for consultancy from Novartis and Biogen, and as a speaker from Merck Serono and Teva. Annovazzi P: received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Merck, Biogen, Teva, Sanofi-Genzyme, Mylan, Almirall, Roche, and Novartis. Capobianco M: received personal compensation for speaking honoraria or participating in advisory boards from: Almirall, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. Signoriello E: received travel funding and speaker honoraria from Biogen, Novartis, Sanofi Genzyme, Bayer, and Teva. Laroni A: received personal compensation from Novartis, Genzyme, Biogen, Merck and TEVA for public speaking and/or advisory boards. La Gioia S: received grants from Novartis. Sartori A: received funding for travel and/or speaker honoraria from Novartis, Teva, Merk, Genzyme, Almirall, and Roche. Maniscalco GT: has served on advisory boards and/or received travel grants and speaker honoraria from Almirall, Biogen, Merck Serono, Novartis and Teva. Bonavita S: received speaker honoraria and advisory board fee from Teva, Genzyme, Biogen, Merck Serono, Novartis, Roche, and Almirall. Clerico M: received personal compensations for participating in advisory boards, public speaking, editorial commitments or travel grants from Biogen Idec, Merck Serono, Fondazione Serono, Novartis, Pomona, Sanofi-Genzyme, and Teva. Russo CV: participated in scientific advisory boards for Merk Serono and Sanofi Genzyme. Gallo A: received travel grants and consulting fees from Biogen, Merck Serono, Roche, Sanofi-Genzyme, and Teva. Lapucci C: has received travel funds from Roche. Carotenuto A: received unrestricted grant from Almirall. Sormani MP: received consulting fees from TEVA, Biogen, Merck, Sanofi Genzyme, Roche, GeNeuro, Novartis, Medday, Actelion, and Celgene. Cocco E: has received honoraria for consultancy or speaking from Bayer, Biogen, Novartis, Sanofi, Genzyme, Merck, and Teva.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Frau, J., Saccà, F., Signori, A. et al. Outcomes after fingolimod to alemtuzumab treatment shift in relapsing–remitting MS patients: a multicentre cohort study. J Neurol 266, 2440–2446 (2019). https://doi.org/10.1007/s00415-019-09424-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09424-8