Abstract
Background and Objectives
Subject dropout rates in placebo-controlled randomized clinical trials (RCTs) of antipsychotics are high. The missing values due to dropout represent a potential source of bias in clinical trials. We aimed to identify the potential factors affecting subject dropout in atypical antipsychotics RCTs by conducting a meta-analysis.
Methods
Placebo-controlled RCTs for atypical antipsychotics using positive and negative syndrome scale (PANSS) as a psychiatric assessment scale were selected by database search. The potential factors affecting subject dropout, such as publication year, study design, and operational factors, were analyzed by meta-regression.
Results
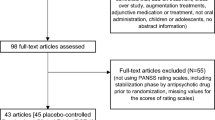
Forty-seven placebo controlled RCTs of atypical antipsychotics of which results were published between 1993 and 2018 were identified through the database search. In the multivariate meta-regression analysis, earlier publication year, older age of subjects, and longer study duration were significantly associated with high subject dropout rates in placebo-controlled clinical trials of atypical antipsychotics.
Conclusion
Subject dropout rates in clinical trials of atypical antipsychotics published between 1993 and 2018 year decreased over time. Study duration should be taken into consideration when designing future studies, where short study periods yet appropriate for evaluating the efficacy of new atypical antipsychotics are preferable. Additionally, previous medications and the degree of subject satisfaction with antipsychotics might affect subject dropout rate.

Similar content being viewed by others
References
Lewis JA, Jonsson B, Kreutz G, Sampaio C, van Zwieten-Boot B. Placebo-controlled trials and the Declaration of Helsinki. Lancet. 2002;359(9314):1337–40. https://doi.org/10.1016/s0140-6736(02)08277-6.
Miller FG. Placebo-controlled trials in psychiatric research: an ethical perspective. Biol Psychiatry. 2000;47(8):707–16.
ICH Harmonised Tripartite Guideline. Statistical principles for clinical trial E9. 1998. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed 27 Jun 2019.
Kemmler G, Hummer M, Widschwendter C, Fleischhacker WW. Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: a meta-analysis. Arch Gen Psychiatry. 2005;62(12):1305–12. https://doi.org/10.1001/archpsyc.62.12.1305.
Wahlbeck K, Tuunainen A, Ahokas A. Dropout rates in randomised antipsychotic drug trials. Psychopharmacology. 2001;155(3):230–3. https://doi.org/10.1007/s0021301007.
Rabinowitz J, Levine SZ, Barkai O, Davidov O. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–88. https://doi.org/10.1093/schbul/sbn005.
Bell M, Milstein R, Beam-Goulet J, Lysaker P, Cicchetti D. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale. Reliability, comparability, and predictive validity. J Nerv Ment Dis. 1992;180(11):723–8.
Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. https://doi.org/10.1093/schbul/13.2.261.
Overall JE. Commentary on the BPRS by John Overall in 1978 at Citation Classics. 1979. http://www.garfield.library.upenn.edu/classics1979/A1979HZ19700001.pdf. Accessed Apr 2017.
Matsusaki A, Kaneko M, Narukawa M. Meta-analysis of placebo response in randomized clinical trials of antipsychotic drugs using PANSS focusing on different approaches to the handling of missing data. Clin Drug Investig. 2018;38(8):751–61. https://doi.org/10.1007/s40261-018-0661-1.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009. https://doi.org/10.1136/bmj.b2700.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2017-04-21. 2017. https://www.R-project.org/. Accessed Apr 2017.
Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol. 1993;13(1):25–40.
Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825–35. https://doi.org/10.1176/ajp.151.6.825.
Beasley CM Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology (Berl). 1996;124(1–2):159–67.
van Kammen DP, McEvoy JP, Targum SD, Kardatzke D, Sebree TB. A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl). 1996;124(1–2):168–75.
Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20(5):491–505. https://doi.org/10.1016/s0893-133x(98)00090-6.
Truffinet P, Tamminga CA, Fabre LF, Meltzer HY, Riviïre M-E, Papillon-Downey C. Placebo-controlled study of the D4/5-HT2A antagonist fananserin in the treatment of schizophrenia. Am J Psychiatry. 1999;156(3):419–25. https://doi.org/10.1176/ajp.156.3.419.
Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–71.
Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–56.
Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–90. https://doi.org/10.1001/archpsyc.60.7.681.
Potkin SG, Gharabawi GM, Greenspan AJ, Mahmoud R, Kosik-Gonzalez C, Rupnow MF, et al. A double-blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencing an acute exacerbation requiring hospitalization. Schizophr Res. 2006;85(1–3):254–65. https://doi.org/10.1016/j.schres.2006.03.027.
Davidson M, Emsley R, Kramer M, Ford L, Pan G, Lim P, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1–3):117–30. https://doi.org/10.1016/j.schres.2007.03.003.
Kahn RS, Schulz SC, Palazov VD, Reyes EB, Brecher M, Svensson O, et al. Efficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):832–42.
Kane J, Canas F, Kramer M, Ford L, Gassmann-Mayer C, Lim P, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1–3):147–61. https://doi.org/10.1016/j.schres.2006.09.012.
Marder SR, Kramer M, Ford L, Eerdekens E, Lim P, Eerdekens M, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–70. https://doi.org/10.1016/j.biopsych.2007.01.017.
McEvoy JP, Daniel DG, Carson WH Jr, McQuade RD, Marcus RN. A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res. 2007;41(11):895–905. https://doi.org/10.1016/j.jpsychires.2007.05.002.
Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68(10):1492–500.
Casey DE, Sands EE, Heisterberg J, Yang HM. Efficacy and safety of bifeprunox in patients with an acute exacerbation of schizophrenia: results from a randomized, double-blind, placebo-controlled, multicenter, dose-finding study. Psychopharmacology (Berl). 2008;200(3):317–31. https://doi.org/10.1007/s00213-008-1207-7.
Cutler AJ, Kalali AH, Weiden PJ, Hamilton J, Wolfgang CD. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 Suppl 1):S20–8. https://doi.org/10.1097/jcp.0b013e318169d4ce.
Potkin SG, Litman RE, Torres R, Wolfgang CD. Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol. 2008;28(2 Suppl 1):S4–11. https://doi.org/10.1097/jcp.0b013e3181692787.
Canuso CM, Dirks B, Carothers J, Kosik-Gonzalez C, Bossie CA, Zhu Y, et al. Randomized, double-blind, placebo-controlled study of paliperidone extended-release and quetiapine in inpatients with recently exacerbated schizophrenia. Am J Psychiatry. 2009;166(6):691–701. https://doi.org/10.1176/appi.ajp.2009.08040613.
Nakamura M, Ogasa M, Guarino J, Phillips D, Severs J, Cucchiaro J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829–36. https://doi.org/10.4088/jcp.08m04905.
Cutler AJ, Tran-Johnson T, Kalali A, Astrom M, Brecher M, Meulien D. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37–69.
Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106–15. https://doi.org/10.1097/jcp.0b013e3181d35d6b.
Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, et al. A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31(3):349–55. https://doi.org/10.1097/jcp.0b013e318218dcd5.
Meltzer HY, Cucchiaro J, Silva R, Ogasa M, Phillips D, Xu J, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957–67. https://doi.org/10.1176/appi.ajp.2011.10060907.
Redden L, Rendenbach-Mueller B, Abi-Saab WM, Katz DA, Goenjian A, Robieson WZ, et al. A double-blind, randomized, placebo-controlled study of the dopamine D(3) receptor antagonist ABT-925 in patients with acute schizophrenia. J Clin Psychopharmacol. 2011;31(2):221–5. https://doi.org/10.1097/jcp.0b013e31820e4818.
Schmidt ME, Kent JM, Daly E, Janssens L, Van Osselaer N, Husken G, et al. A double-blind, randomized, placebo-controlled study with JNJ-37822681, a novel, highly selective, fast dissociating D(2) receptor antagonist in the treatment of acute exacerbation of schizophrenia. Eur Neuropsychopharmacol. 2012;22(10):721–33. https://doi.org/10.1016/j.euroneuro.2012.02.007.
Egan MF, Zhao X, Smith A, Troyer MD, Uebele VN, Pidkorytov V, et al. Randomized controlled study of the T-type calcium channel antagonist MK-8998 for the treatment of acute psychosis in patients with schizophrenia. Hum Psychopharmacol. 2013;28(2):124–33. https://doi.org/10.1002/hup.2289.
Loebel A, Cucchiaro J, Sarma K, Xu L, Hsu C, Kalali AH, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–9. https://doi.org/10.1016/j.schres.2013.01.009.
Nasrallah HA, Silva R, Phillips D, Cucchiaro J, Hsu J, Xu J, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–7. https://doi.org/10.1016/j.jpsychires.2013.01.020.
Ogasa M, Kimura T, Nakamura M, Guarino J. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225(3):519–30. https://doi.org/10.1007/s00213-012-2838-2.
Bugarski-Kirola D, Wang A, Abi-Saab D, Blattler T. A phase II/III trial of bitopertin monotherapy compared with placebo in patients with an acute exacerbation of schizophrenia—results from the CandleLyte study. Eur Neuropsychopharmacol. 2014;24(7):1024–36. https://doi.org/10.1016/j.euroneuro.2014.03.007.
Downing AM, Kinon BJ, Millen BA, Zhang L, Liu L, Morozova MA, et al. A Double-Blind, Placebo-Controlled Comparator Study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry. 2014;14:351. https://doi.org/10.1186/s12888-014-0351-3.
Durgam S, Starace A, Li D, Migliore R, Ruth A, Nemeth G, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–7. https://doi.org/10.1016/j.schres.2013.11.041.
Shen JH, Zhao Y, Rosenzweig-Lipson S, Popp D, Williams JB, Giller E, et al. A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res. 2014;53:14–22. https://doi.org/10.1016/j.jpsychires.2014.02.012.
Correll CU, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–80. https://doi.org/10.1176/appi.ajp.2015.14101275.
Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry. 2015;76(12):e1574–82. https://doi.org/10.4088/jcp.15m09997.
Kane JM, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1–3):127–35. https://doi.org/10.1016/j.schres.2015.01.038.
Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367–73. https://doi.org/10.1097/jcp.0000000000000346.
Potkin SG, Kimura T, Guarino J. A 6-week, double-blind, placebo- and haloperidol-controlled, phase II study of lurasidone in patients with acute schizophrenia. Ther Adv Psychopharmacol. 2015;5(6):322–31. https://doi.org/10.1177/2045125315606027.
Kinoshita T, Bai YM, Kim JH, Miyake M, Oshima N. Efficacy and safety of asenapine in Asian patients with an acute exacerbation of schizophrenia: a multicentre, randomized, double-blind, 6-week, placebo-controlled study. Psychopharmacology (Berl). 2016;233(14):2663–74. https://doi.org/10.1007/s00213-016-4295-9.
Landbloom R, Mackle M, Wu X, Kelly L, Snow-Adami L, McIntyre RS, et al. Asenapine for the treatment of adults with an acute exacerbation of schizophrenia: results from a randomized, double-blind, fixed-dose, placebo-controlled trial with olanzapine as an active control. CNS Spectr. 2016. https://doi.org/10.1017/s1092852916000377.
Lieberman JA, Davis RE, Correll CU, Goff DC, Kane JM, Tamminga CA, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952–61. https://doi.org/10.1016/j.biopsych.2015.08.026.
Cantillon M, Prakash A, Alexander A, Ings R, Sweitzer D, Bhat L. Dopamine serotonin stabilizer RP5063: a randomized, double-blind, placebo-controlled multicenter trial of safety and efficacy in exacerbation of schizophrenia or schizoaffective disorder. Schizophr Res. 2017;189:126–33. https://doi.org/10.1016/j.schres.2017.01.043.
Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2018;72(9):692–700. https://doi.org/10.1111/pcn.12682.
Gharabawi GM, Greenspan A, Rupnow MF, Kosik-Gonzalez C, Bossie CA, Zhu Y, et al. Reduction in psychotic symptoms as a predictor of patient satisfaction with antipsychotic medication in schizophrenia: data from a randomized double-blind trial. BMC Psychiatry. 2006;6:45. https://doi.org/10.1186/1471-244x-6-45.
Schoemaker JH, Vingerhoets A, Emsley RA. Factors associated with poor satisfaction with treatment and trial discontinuation in chronic schizophrenia. CNS Spectr. 2018. https://doi.org/10.1017/s109285291700044x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest that are directly relevant to this research. Akiko Matsusaki is an employee of Chugai Pharmaceutical Co., Ltd.
Funding
The preparation of this manuscript was not supported by any external funding.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsusaki, A., Kaneko, M. & Narukawa, M. Meta-analysis of Dropout Rates in Placebo-Controlled Randomized Clinical Trials of Atypical Antipsychotics Assessed by PANSS. Clin Drug Investig 39, 917–926 (2019). https://doi.org/10.1007/s40261-019-00813-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00813-5