Abstract
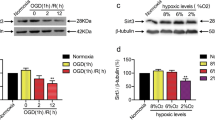
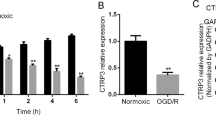
Tripartite motif 32 (TRIM32) is a member of TRIM family that plays a potential role in neural regeneration. However, the biological function of TRIM32 in cerebral ischemia reperfusion injury has not been investigated. In the present study, we evaluated the expression level of TRIM32 in hippocampal neurons following oxygen–glucose deprivation/reperfusion (OGD/R). The results showed that TRIM32 expression was significantly elevated in hippocampal neurons subjected to OGD/R as compared to the neurons cultured in the normoxia condition. To further evaluate the role of TRIM32, hippocampal neurons were transfected with TRIM32 small interfering RNA (si-TRIM32) to knock down TRIM32. We found that knockdown of TRIM32 improved cell viability of OGD/R-stimulated hippocampal neurons. Generation of reactive oxygen species was decreased, while contents of superoxide dismutase and glutathione peroxidase were increased after si-TRIM32 transfection. Knockdown of TRIM32 suppressed cell apoptosis, as proved by the increased bcl-2 expression along with decreased bax expression and caspase-3 activity. We also found that TRIM32 knockdown enhanced OGD/R-induced activation of Nrf2 signaling pathway in hippocampal neurons. Furthermore, siRNA-Nrf2 was transfected to knock down Nrf2. SiRNA-Nrf2 transfection reversed the protective effects of TRIM32 knockdown on neurons. These data suggested that knockdown of TRIM32 protected hippocampal neurons from OGD/R-induced oxidative injury through activating Nrf2 signaling pathway.




Similar content being viewed by others
References
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke Lancet 371:1612–1623
Shiber JR, Fontane E, Adewale A (2010) Stroke registry: hemorrhagic vs ischemic strokes. Am J Emerg Med 28:331–333
Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018) Mitochondria in ischemic stroke: new insight and implications. Aging Dis 9:924–937
Xing C, Hayakawa K, Lo EH (2017) Mechanisms, imaging, and therapy in stroke recovery. Transl Stroke Res 8:1–2
Hofmeijer J, van Putten MJ (2012) Ischemic cerebral damage: an appraisal of synaptic failure. Stroke 43:607–615
Watanabe M, Hatakeyama S (2017) TRIM proteins and diseases. J Biochem 161:135–144
Zhang Y, Wu HK, Lv F, Xiao RP (2017) MG53: biological function and potential as a therapeutic target. Mol Pharmacol 92:211–218
Zhou X, Chen M, Wang S, Yu L, Jiang H (2015) MG53 protein: a promising novel therapeutic target for myocardial ischemia reperfusion injury. Int J Cardiol 199:424–425
Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S (2007) Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neurosci 147:53–59
Xiaoli L, Wujun Z, Jing L (2019) Blocking of tripartite motif 8 protects against lipopolysaccharide (LPS)-induced acute lung injury by regulating AMPKα activity. Biochem Biophys Res Commun 508:701–708
Fu Q, Zou MM, Zhu JW, Zhang Y, Chen WJ, Cheng M et al (2017) TRIM32 affects the recovery of motor function following spinal cord injury through regulating proliferation of glia. Oncotarget 8:45380–45390
Shen H, Pan J, Pan L, Zhang N (2013) TRPC6 inhibited NMDA current in cultured hippocampal neurons. Neuromol Med 15:389–395
Li W, Suwanwela NC, Patumraj S (2016) Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res 106:117–127
Zhang W, Wei R, Zhang L, Tan Y, Qian C (2017) Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience 366:95–104
Zhang ZB, Xiong LL, Lu BT, Zhang HX, Zhang P, Wang TH (2017) Suppression of Trim32 enhances motor function repair after traumatic brain injury associated with antiapoptosis. Cell Transpl 26:1276–1285
Zhang J, Hu MM, Wang YY, Shu HB (2012) TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 287:28646–28655
Sato T, Okumura F, Iguchi A, Ariga T, Hatakeyama S (2012) TRIM32 promotes retinoic acid receptor alpha-mediated differentiation in human promyelogenous leukemic cell line HL60. Biochem Biophys Res Commun 417:594–600
Yokota T, Mishra M, Akatsu H, Tani Y, Miyauchi T, Yamamoto T et al (2006) Brain site-specific gene expression analysis in Alzheimer's disease patients. Eur J Clin Invest 36:820–830
Gonzalez-Cano L, Menzl I, Tisserand J, Nicklas S, Schwamborn JC (2018) Parkinson's disease-associated mutant LRRK2-mediated inhibition of miRNA activity is antagonized by TRIM32. Mol Neurobiol 55:3490–3498
Pavlou MAS, Colombo N, Fuertes-Alvarez S, Nicklas S, Cano LG, Marin MC et al (2017) Expression of the Parkinson's disease-associated gene alpha-synuclein is regulated by the neuronal cell fate determinant TRIM32. Mol Neurobiol 54:4257–4270
Hillje AL, Pavlou MA, Beckmann E, Worlitzer MM, Bahnassawy L, Lewejohann L et al (2013) TRIM32-dependent transcription in adult neural progenitor cells regulates neuronal differentiation. Cell Death Dis 4:e976
Liu Y, Wu W, Yang H, Zhou Z, Zhu X, Sun C et al (2017) Upregulated expression of TRIM32 is involved in Schwann cell differentiation, migration and neurite outgrowth after sciatic nerve crush. Neurochem Res 42:1084–1095
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190:255–266
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol 6:524–551
Raedschelders K, Ansley DM, Chen DD (2012) The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther 133:230–255
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Niture SK, Kaspar JW, Shen J, Jaiswal AK (2010) Nrf2 signaling and cell survival. Toxicol Appl Pharmacol 244:37–42
Guo Y, Yu S, Zhang C, Kong AN (2015) Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med 88:337–349
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81702955), the Scientific Research Program Funded by Department of Science and Technology of Shaanxi Province (Nos. 2018JM7066, 2018JM7065, 2018KJXX-34, 2019JZ-38), Scientific Research Program Funded by Shaanxi Provincial Education Department (Nos. G201711840002, G201711840006), the Leading Disciplines Development Government Foundation of Shaanxi Province (No.[2014]3-1001), grant from the Shannxi Key Laboratory of Ischemic Cardiovascular Disease (No. 2017ZDKF01), supporting Program Funded by Xi’an Medical University (Nos. 2018XNRC07, 2018GJFY02, 2017GJFY25, 2016PT06).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, L., Zhang, Js., Ji, Sf. et al. Knockdown of TRIM32 Protects Hippocampal Neurons from Oxygen–Glucose Deprivation-Induced Injury. Neurochem Res 44, 2182–2189 (2019). https://doi.org/10.1007/s11064-019-02857-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02857-7