Abstract
Background/Aims
Nausea is a major complaint of gastroparesis (GP), and the pathophysiology of this condition is poorly understood. Therefore, this study utilized fMRI to investigate the possible central nervous system (CNS) mechanisms of nausea in 10 GP patients versus 8 healthy controls (HCs).
Methods
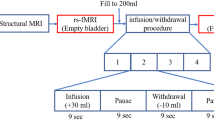
Nausea severity was assessed on a 0–10 scale and presented as mean ± SD. Nausea was increased from baseline utilizing up to 30 min of visual stimulation (VS). Functional network connectivity was measured with fMRI at baseline and after 30 min of VS. fMRI data were preprocessed using statistical parametric mapping software. Thirty-four independent components were identified as meaningful resting-state networks (RSNs) by group independent component analysis. The Functional Network Connectivity (FNC) among 5 RSNs considered important in CNS nausea mechanisms was calculated as the Pearson’s pairwise correlation.
Results
Baseline nausea score in GP patients was 2.7 ± 2.0 and increased to 7.0 ± 1.5 after stimulation (P < 0.01). In HCs nausea scores did not increase from baseline after stimulus (0.3 ± 0.5). When comparing GP patients to HCs after VS, a significant reduction (P < 0.001) in bilateral insula network connectivity compared to the right insula network was detected. No significant differences in connectivity were noted among the other RSNs. Additionally, the average gray matter volume was non-significantly reduced in the insula in GP patients compared to HC.
Conclusions
The insula connectivity network is impaired in nauseated GP patients. This phenomenon could explain the susceptibility of GP patients to nausea or may have resulted from a state of chronic nausea.



Similar content being viewed by others
Abbreviations
- fMRI:
-
Functional magnetic resonance imaging
- FNC:
-
Functional network connectivity
- GP:
-
Gastroparesis
- GICA:
-
Group independent component analysis
- HC:
-
Healthy control
- IC:
-
Independent component
- RSN:
-
Resting-state network
- VS:
-
Visual stimulation
References
Sarosiek I, Song G, Sun Y, et al. Central and peripheral effects of transcutaneous acupuncture treatment for nausea in patients with diabetic gastroparesis. J Neurogastroenterol Motil. 2017;23:245–253.
Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–38.
Parkman H, McCallum R. Gastroparesis: Pathophysiology, Presentation, and Treatment. New York: Humana Press; 2012.
Bashashati M, McCallum RW. Is interstitial cells of cajal-opathy present in gastroparesis? J Neurogastroenterol Motil. 2015;21:486–493.
Napadow V, Sheehan JD, Kim J, et al. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex. 2013;23:806–813.
Toschi N, Kim J, Sclocco R, et al. Motion sickness increases functional connectivity between visual motion and nausea-associated brain regions. Auton Neurosci. 2017;202:108–113.
Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54.
Kiernan BD, Soykan I, Lin Z, Dale A, McCallum RW. A new nausea model in humans produces mild nausea without electrogastrogram and vasopressin changes. Neurogastroenterol Motil. 1997;9:257–263.
Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151.
Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Frontiers Syst Neurosci. 2011;5:2.
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095.
Meda SA, Giuliani NR, Calhoun VD, et al. A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 2008;101:95–105.
Robinson S, Basso G, Soldati N, et al. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:137.
Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333.
Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851.
Funding
The funding for this work was provided by Texas Tech University Health Sciences Center of El Paso Internal Medicine Department Seed Grant and Texas Tech University Health Sciences Center of El Paso, Paul L. Foster School of Medicine Scholarly Activity and Research Program Mini Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Richard McCallum was supported by CinRx Pharma (Consultant or advisor), GI Stimultation-Patent on “gastric pacing” (owner of patent), Allergan Pharma, Takeda Pharma, Vanda Pharma, (Research Support), Salix Pharma (Speaker’s Bureau, Takeda Pharma (Publications, Steering Committee). The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Snodgrass, P., Sandoval, H., Calhoun, V.D. et al. Central Nervous System Mechanisms of Nausea in Gastroparesis: An fMRI-Based Case–Control Study. Dig Dis Sci 65, 551–556 (2020). https://doi.org/10.1007/s10620-019-05766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05766-5