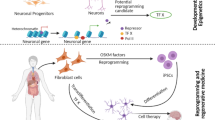
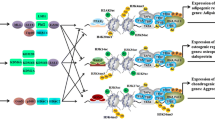
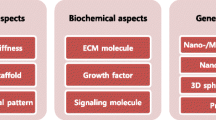
Abstract
Differentiation potential of stem cells into various lineages makes these cells as promising sources to treat multiple diseases. In this regard, the use of different strategies and protocols to increase differentiation capacity is highly demanded. Low-level laser therapy, a relatively noninvasive technique, has the capacity to accelerate the healing of numerous injuries and a portion of restorative capacity could be correlated with the stem cell activation and differentiation. Several mechanisms have been diagnosed to participate in orientation of stem cells to functional mature cells. Among them, the status of DNA methylation orchestrates the maintenance of tissue-specific gene expression during the differentiation procedure. DNA methylation is a momentous event in embryogenesis and functional maturation. This review article highlighted the potency of laser irradiation (low-level intensities) in the differentiation of stem cells by modulation of methylation. The analysis of these modalities could help us to understand the underlying mechanisms participating in the therapeutic effects of photobiomodulation.


Similar content being viewed by others
References
Biehl JK, Russell B (2009) Introduction to stem cell therapy. J Cardiovasc Nurs 24(2):98
Masoud Maleki, (2015) Stem cell therapy of cataract. BioImpacts 5(4):165–167
Alexander MS, Casar JC, Motohashi N (2015) Stem cell differentiation and therapeutic use. Stem Cells Int 2015
Saeed Azandeh, Anneh Mohammad Gharravi, Mahmoud Orazizadeh, Ali Khodadi, Mahmoud Hashemi Tabar, (2016) Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial. BioImpacts 6(1):9–13
Nadig RR (2009) Stem cell therapy–hype or hope? A review. J Conserv Dent: JCD 12(4):131
Mohammadian M, Shamsasenjan K, Lotfi nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassagh pour A (2013) Mesenchymal Stem Cells: New Aspect in Cell-Based Regenerative Therapy. Adv Pharm Bull 3(2):433–437. https://doi.org/10.5681/apb.2013.070
Keller G (2005) Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 19(10):1129–1155
Elbuluk A, Einhorn TA, Iorio R (2017) A comprehensive review of stem-cell therapy. JBJS Rev 5(8):e15
Bradley A, Evans M, Kaufman MH, Robertson E (1984) Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309(5965):255
Alison M, Islam S (2009) Attributes of adult stem cells. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland 217(2):144–160
Hwang NS, Zhang C, Hwang YS, Varghese S (2009) Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med 1(1):97–106
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA (2013) Mutational landscape and significance across 12 major cancer types. Nature 502(7471):333
Huang K, Fan G (2010) DNA methylation in cell differentiation and reprogramming: an emerging systematic view. Regen Med 5(4):531–544
Kim H, Choi K, Kweon O-K, Kim WH (2012) Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J Dermatol Sci 68(3):149–156
Leonida A, Paiusco A, Rossi G, Carini F, Baldoni M, Caccianiga G (2013) Effects of low-level laser irradiation on proliferation and osteoblastic differentiation of human mesenchymal stem cells seeded on a three-dimensional biomatrix: in vitro pilot study. Lasers Med Sci 28(1):125–132
Abramovitch-Gottlib L, Gross T, Naveh D, Geresh S, Rosenwaks S, Bar I, Vago R (2005) Low level laser irradiation stimulates osteogenic phenotype of mesenchymal stem cells seeded on a three-dimensional biomatrix. Lasers Med Sci 20(3–4):138–146
Mvula B, Abrahamse H (2014) Low intensity laser irradiation and growth factors influence differentiation of adipose derived stem cells into smooth muscle cells in a coculture environment over a period of 72 hours. Int J Photoenergy 2014
Soleimani M, Abbasnia E, Fathi M, Sahraei H, Fathi Y, Kaka G (2012) The effects of low-level laser irradiation on differentiation and proliferation of human bone marrow mesenchymal stem cells into neurons and osteoblasts—an in vitro study. Lasers Med Sci 27(2):423–430
Jf H, Zhang H, Yuan X, Li J, Yj W, Hu S (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery 40(10):726–733
Wang Y, Huang Y-Y, Wang Y, Lyu P, Hamblin MR (2016) Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep 6:33719
Zhang R, Wang Q, Zhang A, Xu J, Zhai L, Yang X, Liu X (2018) Low-level laser irradiation promotes the differentiation of bone marrow stromal cells into osteoblasts through the APN/Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci 22(9):2860–2868
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Ther 23(2):161–166
Kim M, Kang T-W, Lee H-C, Han Y-M, Kim H, Shin HD, Cheong HS, Lee D, Kim S-Y, Kim YS (2011) Identification of DNA methylation markers for lineage commitment of in vitro hepatogenesis. Hum Mol Genet 20(14):2722–2733
Mummery C, Van den Brink C, De Laat S (1987) Commitment to differentiation induced by retinoic acid in P19 embryonal carcinoma cells is cell cycle dependent. Dev Biol 121(1):10–19
Lange C, Calegari F (2010) Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle 9(10):1893–1900
Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125(2):315–326
Voigt P, Tee W-W, Reinberg D (2013) A double take on bivalent promoters. Genes Dev 27(12):1318–1338
Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132(4):567–582
M Perez-Campo F, A Riancho J (2015) Epigenetic mechanisms regulating mesenchymal stem cell differentiation. Curr Genomics 16 (6):368–383
Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA (2010) DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463(7280):563
Smith ZD, Meissner A (2013) DNA methylation: roles in mammalian development. Nat Rev Genet 14(3):204
Berdasco M, Esteller M (2011) DNA methylation in stem cell renewal and multipotency. Stem Cell Res Ther 2(5):42
Boland MJ, Nazor KL, Loring JF (2014) Epigenetic regulation of pluripotency and differentiation. Circ Res 115(2):311–324
Sheaffer KL, Kim R, Aoki R, Elliott EN, Schug J, Burger L, Schübeler D, Kaestner KH (2014) DNA methylation is required for the control of stem cell differentiation in the small intestine. Genes Dev 28(6):652–664
Altun G, Loring JF, Laurent LC (2010) DNA methylation in embryonic stem cells. J Cell Biochem 109(1):1–6
Podobinska M, Szablowska-Gadomska I, Augustyniak J, Sandvig I, Sandvig A, Buzanska L (2017) Epigenetic modulation of stem cells in neurodevelopment: the role of methylation and acetylation. Front Cell Neurosci 11:23
Kim M, Costello J (2017) DNA methylation: an epigenetic mark of cellular memory. Exp Mol Med 49(4):e322
Zhou Y, Kim J, Yuan X, Braun T (2011) Epigenetic modifications of stem cells: a paradigm for the control of cardiac progenitor cells. Circ Res 109(9):1067–1081
Bibikova M, Chudin E, Wu B, Zhou L, Garcia EW, Liu Y, Shin S, Plaia TW, Auerbach JM, Arking DE (2006) Human embryonic stem cells have a unique epigenetic signature. Genome Res 16(9):1075–1083
Cotler HB, Chow RT, Hamblin MR, Carroll J (2015) The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol 2(5)
Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533
Costa MM, Silva SB, Quinto ALP, Pasquinelli PFS, dos Santos VQ, de Cássia Santos G, Veiga DF (2014) Phototherapy 660 nm for the prevention of radiodermatitis in breast cancer patients receiving radiation therapy: study protocol for a randomized controlled trial. Trials 15(1):330
Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A (2014) Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J Lasers Med Sci 5(4):163
Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD (2005) Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery 36(1):8–12
Sibata C, Colussi V, Oleinick N, Kinsella T (2000) Photodynamic therapy: a new concept in medical treatment. Braz J Med Biol Res 33(8):869–880
Hashmi JT, Huang Y-Y, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR (2010) Role of low-level laser therapy in neurorehabilitation. Pm&r 2(12):S292–S305
Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR (2013) Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics 6(10):829–838
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of selected topics in quantum electronics 22(3):348–364
Maraldi T, Angeloni C, Giannoni E, Sell C (2015) Reactive oxygen species in stem cells. Oxidative Med Cell Longev 2015
Nugud A, Sandeep D, El-Serafi AT (2018) Two faces of the coin: minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J Adv Res
Abrahamse H (2012) Regenerative medicine, stem cells, and low-level laser therapy: future directives. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA,
Kushibiki T, Hirasawa T, Okawa S, Ishihara M (2015) Low reactive level laser therapy for mesenchymal stromal cells therapies. Stem Cells Int 2015
Fekrazad R, Asefi S, Allahdadi M, Kalhori KA (2016) Effect of photobiomodulation on mesenchymal stem cells. Photomed Laser Surg 34(11):533–542
de Matos Gomes MV, Manfredo MH, Toffoli LV, Castro-Alves DC, do Nascimento LM, da Silva WR, Kashimoto RK, Rodrigues-Jr GM, Estrada VB, Andraus RA (2016) Effects of the led therapy on the global DNA methylation and the expression of Dnmt1 and Dnmt3a genes in a rat model of skin wound healing. Lasers Med Sci 31(7):1521–1526
Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K (1996) Studies of the mortality of atomic bomb survivors. Report 12, part I. Cancer: 1950-1990. Radiat Res 146(1):1–27
Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K (2003) Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 160(4):381–407
Warner JK, Wang JC, Hope KJ, Jin L, Dick JE (2004) Concepts of human leukemic development. Oncogene 23(43):7164
de Farias GA, Wagner V, Correa C, Webber L, Pilar E, Curra M, Carrard V, Martins M, Martins M (2019) Photobiomodulation therapy modulates epigenetic events and NF-κB expression in oral epithelial wound healing. Lasers Med Sci
Acknowledgments
We would thank the personnel of Stem Cell Research Center for collaboration.
Funding
This manuscript is supported by a grant from Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
A.R.N.Z., S.S., M.H.G., F.B., and L.H. collected the data and wrote manuscript. R. R. conducted the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zamani, A.R.N., Saberianpour, S., Geranmayeh, M.H. et al. Modulatory effect of photobiomodulation on stem cell epigenetic memory: a highlight on differentiation capacity. Lasers Med Sci 35, 299–306 (2020). https://doi.org/10.1007/s10103-019-02873-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02873-7