Abstract
Introduction
Matrix damage sustained by bone tissue is repaired by the concerted action of bone cells. Previous studies have reported extracellular calcium ([Ca2+]E) efflux to originate from regions of bone undergoing diffuse microdamage termed as “diffuse microdamage-induced calcium efflux” (DMICE). DMICE has also been shown to activate and increase intracellular calcium ([Ca2+]I) signaling in osteoblasts via the involvement of voltage-gated calcium channels (VGCC). Past studies have assessed early stage (< 1 h) responses of osteoblasts to DMICE. The current study tested the hypothesis that DMICE has longer-term sustained effect such that it induces anabolic response of osteoblasts.
Materials and methods
Osteoblasts derived from mouse calvariae were seeded on devitalized bovine bone wafers. Localized diffuse damage was induced in the vicinity of cells by bending. The response of osteoblasts to DMICE was evaluated by testing gene expression, protein synthesis and mineralized nodule formation.
Results
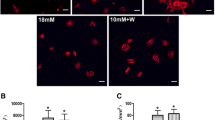
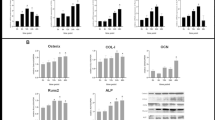
Cells on damaged bone wafers showed a significant increase in RUNX2 and Osterix expression compared to non-loaded control. Also, RUNX2 and Osterix expression were suppressed significantly when the cells were treated with bepridil, a non-selective VGCC inhibitor, prior to loading. Significantly higher amounts of osteocalcin and mineralized nodules were synthesized by osteoblasts on diffuse damaged bone wafers, while bepridil treatment resulted in a significant decrease in osteocalcin production and mineralized nodule formation.
Conclusion
In conclusion, this study demonstrated that DMICE activates anabolic responses of osteoblasts through activation of VGCC. Future studies of osteoblast response to DMICE in vivo will help to clarify how bone cells repair diffuse microdamage.





Similar content being viewed by others
References
Gupta HS, Zioupos P (2008) Fracture of bone tissue: the ‘hows’ and the ‘whys’. Med Eng Phys 30:1209–1226
Frost H (1960) Presence of microscopic cracks in vivo in bone. Henry Ford Hosp Med Bull 8:35
Sahar ND, Hong SI, Kohn DH (2005) Micro- and nano-structural analyses of damage in bone. Micron 36:617–629
Seref-Ferlengez Z, Kennedy OD, Schaffler MB (2015) Bone microdamage, remodeling and bone fragility: how much damage is too much damage? Bonekey Rep 4:644
Wasserman N, Brydges B, Searles S, Akkus O (2008) In vivo linear microcracks of human femoral cortical bone remain parallel to osteons during aging. Bone 43:856–861
Carter DR, Hayes WC (1977) Compact bone fatigue damage: a microscopic examination. Clin Orthop Relat Res 127:265–274
Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R (1998) Does microdamage accumulation affect the mechanical properties of bone? J Biomech 31:337–345
Sobelman OS, Gibeling JC, Stover SM, Hazelwood SJ, Yeh OC, Shelton DR, Martin RB (2004) Do microcracks decrease or increase fatigue resistance in cortical bone? J Biomech 37:1295–1303
Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB (2009) Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 24:597–605
Taylor D, Hazenberg JG, Lee TC (2007) Living with cracks: damage and repair in human bone. Nat Mater 6:263–268
Lee TC, Staines A, Taylor D (2002) Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat 201:437–446
Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB (2010) Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone 47:766–772
Yan YX, Gong YW, Guo Y, Lv Q, Guo C, Zhuang Y, Zhang Y, Li R, Zhang XZ (2012) Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation. PLoS One 7:e35709
Guo Y, Zhang CQ, Zeng QC, Li RX, Liu L, Hao QX, Shi CH, Zhang XZ, Yan YX (2012) Mechanical strain promotes osteoblast ECM formation and improves its osteoinductive potential. Biomed Eng Online 11:80
Letechipia JE, Alessi A, Rodriguez G, Asbun J (2010) Would increased interstitial fluid flow through in situ mechanical stimulation enhance bone remodeling? Med Hypotheses 75:196–198
Turner CH, Forwood MR, Otter MW (1994) Mechanotransduction in bone: do bone cells act as sensors of fluid flow? FASEB J 8:875–878
Sun X, McLamore E, Kishore V, Fites K, Slipchenko M, Porterfield DM, Akkus O (2012) Mechanical stretch induced calcium efflux from bone matrix stimulates osteoblasts. Bone 50:581–591
Sun X, Hoon Jeon J, Blendell J, Akkus O (2010) Visualization of a phantom post-yield deformation process in cortical bone. J Biomech 43:1989–1996
Jung H, Best M, Akkus O (2015) Microdamage induced calcium efflux from bone matrix activates intracellular calcium signaling in osteoblasts via L-type and T-type voltage-gated calcium channels. Bone 76:88–96
Yamauchi M, Yamaguchi T, Kaji H, Sugimoto T, Chihara K (2005) Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am J Physiol Endocrinol Metab 288:E608–E616
Rosen LB, Ginty DD, Weber MJ, Greenberg ME (1994) Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12:1207–1221
Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR (2005) Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech 38:1909–1917
Jung H, Mbimba T, Unal M, Akkus O (2018) Repetitive short-span application of extracellular calcium is osteopromotive to osteoprogenitor cells. J Tissue Eng Regen Med 12:e1349–e1359
Jung H, Akkus O (2016) Activation of intracellular calcium signaling in osteoblasts colocalizes with the formation of post-yield diffuse microdamage in bone matrix. Bonekey Rep 5:778
Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB (1998) Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res 16:322–329
Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R (2012) Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One 7:e34980
Lynch JA, Silva MJ (2008) In vivo static creep loading of the rat forelimb reduces ulnar structural properties at time-zero and induces damage-dependent woven bone formation. Bone 42:942–949
Seref-Ferlengez Z, Basta-Pljakic J, Kennedy OD, Philemon CJ, Schaffler MB (2014) Structural and mechanical repair of diffuse damage in cortical bone in vivo. J Bone Miner Res 29:2537–2544
Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, Fyhrie DP (2000) In vivo diffuse damage in human vertebral trabecular bone. Bone 26:147–152
Cianferotti L, Gomes AR, Fabbri S, Tanini A, Brandi ML (2015) The calcium-sensing receptor in bone metabolism: from bench to bedside and back. Osteoporos Int 26:2055–2071
Gu Y, Preston MR, Magnay J, El Haj AJ, Publicover SJ (2001) Hormonally-regulated expression of voltage-operated Ca(2+) channels in osteocytic (MLO-Y4) cells. Biochem Biophys Res Commun 282:536–542
Bonewald LF (2007) Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci 1116:281–290
Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GA, Stucky GD, Morse DE, Hansma PK (2005) Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 4:612–616
Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK (2001) Bone indentation recovery time correlates with bond reforming time. Nature 414:773–776
Gupta HS, Wagermaier W, Zickler GA, Raz-Ben Aroush D, Funari SS, Roschger P, Wagner HD, Fratzl P (2005) Nanoscale deformation mechanisms in bone. Nano Lett 5:2108–2111
Lohberger B, Kaltenegger H, Stuendl N, Payer M, Rinner B, Leithner A (2014) Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. Biomed Res Int 2014:189516
Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2007) Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 101:1266–1277
Lu XL, Huo B, Park M, Guo XE (2012) Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone 51:466–473
Cullen PJ, Lockyer PJ (2002) Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol 3:339–348
Seo JH, Jin YH, Jeong HM, Kim YJ, Jeong HG, Yeo CY, Lee KY (2009) Calmodulin-dependent kinase II regulates Dlx5 during osteoblast differentiation. Biochem Biophys Res Commun 384:100–104
Samee N, Geoffroy V, Marty C, Schiltz C, Vieux-Rochas M, Levi G, de Vernejoul MC (2008) Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am J Pathol 173:773–780
Komori T (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339:189–195
Komori T (2005) Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem 95:445–453
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ (2002) Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Investig 109:1405–1415
Choudhary S, Kumar A, Kale RK, Raisz LG, Pilbeam CC (2004) Extracellular calcium induces COX-2 in osteoblasts via a PKA pathway. Biochem Biophys Res Commun 322:395–402
Yamaguchi T, Chattopadhyay N, Kifor O, Sanders JL, Brown EM (2000) Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem Biophys Res Commun 279:363–368
Choi YH, Gu YM, Oh JW, Lee KY (2011) Osterix is regulated by Erk1/2 during osteoblast differentiation. Biochem Biophys Res Commun 415:472–478
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, Darnay BG, de Crombrugghe B (2010) Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci USA 107:12919–12924
Acknowledgements
This study was funded by the National Science Foundation (NSF CMMI-1233413).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Ethical approval
Animals were used in accordance with protocol (2014-0099) approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Jung, H., Akkus, O. Diffuse microdamage in bone activates anabolic response by osteoblasts via involvement of voltage-gated calcium channels. J Bone Miner Metab 38, 151–160 (2020). https://doi.org/10.1007/s00774-019-01042-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-01042-8