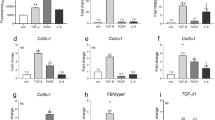
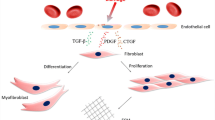
Abstract
Skin fibrosis, characterized by excessive fibroblast proliferation and extracellular matrix deposition in the dermis, is the histopathologic hallmark of dermatologic diseases such as systemic sclerosis, hypertrophic scars, and keloids. Effective anti-scarring therapeutics remain an unmet need, underscoring the complex pathophysiologic mechanisms of skin fibrosis. The Th2 cytokines interleukin (IL)-4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders. The goal of this article is to summarize the current understanding of the role of the IL-4/IL-13 axis in wound healing and skin fibrosis. We conducted a literature search to identify research studies investigating the roles of IL-4 and IL-13 in fibrotic skin diseases. While transforming growth factor-beta has long been regarded as the main driver of fibrotic processes, research into the cellular and molecular biology of wound healing has revealed other pathways that promote scar tissue formation. IL-4 and IL-13 are important mediators of skin fibrosis, supported by evidence from in vitro data, animal models of fibrosis, and clinical studies. Overactive signaling of the IL-4/IL-13 axis contributes to the initiation and perpetuation of fibrotic skin diseases. Further insights into the IL-4/IL-13 axis may reveal potential targets for the development of novel therapies that prevent or treat fibrotic skin diseases.




Similar content being viewed by others
References
Allanore Y, Matucci-Cerinic M, Distler O (2016) Treatment of systemic sclerosis: is there any hope for the future? RMD Open 2:e000260
Allen JE, Wynn TA (2011) Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog 7:5–8
Altair Therapeutics. Study evaluating the effects of AIR645 on allergen-induced airway responses in subjects with mild atopic asthma. https://clinicaltrials.gov/ct2/show/NCT00941577. Accessed 10 Nov 2018
Artlett CM (2014) Animal models of systemic sclerosis: their utility and limitations. Open Access Rheumatol Res Rev 6:65–81
AstraZeneca (2018) Clinical trials appendix Q1 2018 results update. https://www.astrazeneca.com/content/dam/az/PDF/2018/Q1-2018/Q1 2018 Clinical trials appendix.pdf. Accessed 10 Nov 2018
Bachert C, Mannent L, Naclerio RM et al (2016) Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis. JAMA 315:469
Bagnasco D, Ferrando M, Varricchi G et al (2016) A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol 170:122–131
Bayat A, McGrouther D, Ferguson M (2003) Skin scarring. BMJ 326:88–92
Beyer C, Schett G, Distler O, Distler JHW (2010) Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum 62:2831–2844
Bhogal RK, Stoica CM, McGaha TL, Bona CA (2005) Molecular aspects of regulation of collagen gene expression in fibrosis. J Clin Immunol 25:592–603
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433–438
De Boever EH, Ashman C, Cahn AP et al (2014) Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol 133:989.e4–996.e4
Borish LC, Nelson HS, Lanz MJ et al (1999) Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med 160:1816–1823
Borthwick LA, Wynn TA, Fisher AJ (2013) Cytokine mediated tissue fibrosis. Biochim Biophys Acta Mol Basis Dis 1832:1049–1060
Brown BC, McKenna SP, Siddhi K et al (2008) The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthetic Surg 61:1049–1058
Brunner SM, Schiechl G, Kesselring R et al (2013) IL-13 signaling via IL-13Rα2 triggers TGF-β1-dependent allograft fibrosis. Transplant Res 2:16
Bush JA, McGrouther DA, Young VL et al (2011) Recommendations on clinical proof of efficacy for potential scar prevention and reduction therapies. Wound Repair Regen 19:s32–s37
Catley MC (2010) Asthma and COPD–IQPC’s second conference. IDrugs 13:601–604
Chaker AM, Shamji MH, Dumitru FA et al (2016) Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: a randomized controlled trial. J Allergy Clin Immunol 137:452.e9–461.e9
Chiaramonte MG, Mentink-Kane M, Jacobson BA et al (2003) Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med 197:687–701
Chomarat P, Banchereau J (1998) Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol 17:1–52
Corren J, Busse W, Meltzer EO et al (2010) A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med 181:788–796
Danese S, Rudziński J, Brandt W et al (2015) Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 64:243–249
Darby IA, Zakuan N, Billet F, Desmoulière A (2016) The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 73:1145–1157
Darby IA, Laverdet B, Bonté F, Desmoulière A (2014) Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7:301–311
Denton CP, Abraham DJ (2004) Transgenic analysis of scleroderma: understanding key pathogenic events in vivo. Autoimmun Rev 3:285–293
Elbe-Bürger A, Egyed A, Olt S et al (2002) Overexpression of IL-4 alters the homeostasis in the skin. J Investig Dermatol 118:767–778
Fertin C, Nicolas JF, Gillery P et al (1991) Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol 37:823–829
Fichtner-Feigl S, Strober W, Kawakami K et al (2006) IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med 12:99–106
Fuschiotti P (2011) Role of IL-13 in systemic sclerosis. Cytokine 56:544–549
Fuschiotti P, Larregina AT, Ho J et al (2013) Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 65:236–246
Fuschiotti P, Medsger TAJ, Morel PA (2009) Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum 60:1119–1128
Gandhi NA, Pirozzi G, Graham NMH (2017) Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 13:425–437
Gause WC, Wynn TA, Allen JE (2013) Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 13:607–614
Gauvreau GM, Boulet L-P, Cockcroft DW et al (2011) Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med 183:1007–1014
Gieseck RL, Wilson MS, Wynn TA (2018) Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18:62–76
Gillery P, Fertin C, Nicolas JF et al (1992) Interleukin-4 stimulates collagen gene expression in human fibroblast monolayer cultures. Potential role in fibrosis. FEBS Lett 302:231–234
Gold MH, Berman B, Clementoni MT et al (2014) Updated international clinical recommendations on scar management: part 1—evaluating the evidence. Dermatol Surg 40:817–824
Gold MH, McGuire M, Mustoe TA et al (2014) Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg 40:825–831
Greenblatt MB, Aliprantis AO (2013) The immune pathogenesis of scleroderma: context is everything. Curr Rheumatol Rep 15:297
Greenblatt MB, Sargent JL, Farina G et al (2012) Interspecies comparison of human and murine scleroderma reveals IL-13 and CCL2 as disease subset-specific targets. Am J Pathol 180:1080–1094
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Guttman E (2018) A pilot study of tralokinumab in subjects with moderate to severe alopecia areata. https://clinicaltrials.gov/ct2/show/NCT02684097. Accessed 10 Nov 2018
Hanania NA, Korenblat P, Chapman KR et al (2016) Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 4:781–796
Hanania NA, Noonan M, Corren J et al (2015) Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 70:748–756
Hart TK, Blackburn MN, Brigham-Burke M et al (2002) Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol 130:93–100
Hasegawa M, Fujimoto M, Kikuchi K, Takehara K (1997) Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol 24:328–332
Hasegawa M, Sato S, Nagaoka T et al (2003) Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology 207:141–147
He W, Dai C (2015) Key fibrogenic signaling. Curr Pathobiol Rep 3:183–192
Hirano I, Collins MH, Assouline-Dayan Y et al (2019) RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 156:592–603.e10
Hoffmann KF, McCarty TC, Segal DH et al (2001) Disease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactions. FASEB J 15:2545–2547
Hua F, Ribbing J, Reinisch W et al (2015) A pharmacokinetic comparison of anrukinzumab, an anti- IL-13 monoclonal antibody, among healthy volunteers, asthma and ulcerative colitis patients. Br J Clin Pharmacol 80:101–109
Huang X-L, Wang Y-J, Yan J-W et al (2015) Role of anti-inflammatory cytokines IL-4 and IL-13 in systemic sclerosis. Inflamm Res 64:151–159
Ihn H, Yamane K, Kubo M, Tamaki K (2001) Blockade of endogenous transforming growth factor beta signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor beta receptors. Arthritis Rheum 44:474–480
Jagdeo J, Shumaker PR (2017) Traumatic scarring. JAMA Dermatol 153:364
Jinnin M, Ihn H, Yamane K, Tamaki K (2004) Interleukin-13 stimulates the transcription of the human 2(I) collagen gene in human dermal fibroblasts. J Biol Chem 279:41783–41791
Kaufman BP, Alexis AF (2018) Biologics and small molecule agents in allergic and immunologic skin diseases. Curr Allergy Asthma Rep 18:55
Kaviratne M, Hesse M, Leusink M et al (2004) IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol 173:4020–4029
Knipper JA, Willenborg S, Brinckmann J et al (2015) Interleukin-4 receptor α signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 43:803–816
Kodera T, McGaha TL, Phelps R et al (2002) Disrupting the IL-4 gene rescues mice homozygous for the tight-skin mutation from embryonic death and diminishes TGF-beta production by fibroblasts. Proc Natl Acad Sci USA 99:3800–3805
Landén NX, Li D, Ståhle M (2016) Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci 73:3861–3885
Laurent P, Jolivel V, Manicki P et al (2017) Immune-mediated repair: a matter of plasticity. Front Immunol 8:1–8
Lawrence MG, Steinke JW, Borish L (2018) Cytokine-targeting biologics for allergic diseases. Ann Allergy Asthma Immunol 120:376–381
Leask A, Abraham DJ (2004) TGF-β signaling and the fibrotic response. FASEB J 18:816–827
Lee CG, Homer RJ, Zhu Z et al (2001) Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med 194:809–821
Lee H, Jang Y (2018) Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci 19:711
Lee KS, Ro YJ, Ryoo YW et al (1996) Regulation of interleukin-4 on collagen gene expression by systemic sclerosis fibroblasts in culture. J Dermatol Sci 12:110–117
Legrand F, Klion AD (2015) Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract 3:167–174
LEO Pharma. Tralokinumab monotherapy for moderate to severe atopic dermatitis—ECZTRA 1 (ECZema TRAlokinumab trial no. 1) (ECZTRA 1). https://clinicaltrials.gov/ct2/show/NCT03131648. Accessed 10 Nov 2018
LEO Pharma. Long-term extension trial in subjects with atopic dermatitis who participated in previous tralokinumab trials—ECZTEND. https://clinicaltrials.gov/ct2/show/NCT03587805. Accessed 10 Nov 2018
Liang H, Zhang Z, Yan J et al (2017) The IL-4 receptor α has a critical role in bone marrow-derived fibroblast activation and renal fibrosis. Kidney Int 92:1433–1443
Lichtman MK, Otero-Vinas M, Falanga V (2016) Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen 24:215–222
Lloyd CM, Snelgrove RJ (2018) Type 2 immunity: expanding our view. Sci Immunol 3:1604
Loke P, Gallagher I, Nair MG et al (2007) Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol 179:3926–3936
Lucey DR, Clerici M, Shearer GM (1996) Type 1, and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev 9:532–562
Luzina IG, Atamas SP (2008) Fibrotic skin diseases. In: Gaspari A, Tyring S (eds) Clinical and basic immunodermatology. Springer, London, pp 721–737
MacDonald TT (2006) Decoy receptor springs to life and eases fibrosis. Nat Med 12:13–14
Maes T, Joos GF, Brusselle GG (2012) Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol 47:261–270
Marshall CD, Hu MS, Leavitt T et al (2018) Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care 7:29–45
May RD, Fung M (2015) Strategies targeting the IL-4/IL-13 axes in disease. Cytokine 75:89–116
McCormick LL, Zhang Y, Tootell E, Gilliam AC (1999) Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol 163:5693–5699
McCormick SM, Heller NM (2015) Commentary: iL-4 and IL-13 receptors and signaling. Cytokine 75:38–50
McGaha T, Saito S, Phelps RG et al (2001) Lack of skin fibrosis in tight skin (TSK) mice with targeted mutation in the interleukin-4R alpha and transforming growth factor-beta genes. J Investig Dermatol 116:136–143
McGaha TL, Le M, Kodera T et al (2003) Molecular mechanisms of interleukin-4-induced up-regulation of type I collagen gene expression in murine fibroblasts. Arthritis Rheum 48:2275–2284
McGaha TL, Bona CA (2002) Role of profibrogenic cytokines secreted by T cells in fibrotic processes in scleroderma. Autoimmun Rev 1:174–181
Memorial Sloan Kettering Cancer Center. Immunotherapy for the treatment of breast cancer related upper extremity lymphedema (BCRL). https://clinicaltrials.gov/ct2/show/NCT02494206. Accessed 10 Nov 2018
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Le Moine A, Flamand V, Demoor FX et al (1999) Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. J Clin Investig 103:1659–1667
Mokos ZB, Jović A, Grgurević L et al (2017) Current therapeutic approach to hypertrophic scars. Front Med 4:1–11
Needleman BW, Wigley FM, Stair RW (1992) Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum 35:67–72
Nicholson GC, Kariyawasam HH, Tan AJ et al (2011) The effects of an anti–IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J Allergy Clin Immunol 128:800.e9–807.e9
Novartis Pharmaceuticals. A phase II efficacy study in fistulizing Crohn’s disease patients. https://clinicaltrials.gov/ct2/show/NCT01355614. Accessed 10 Nov 2018
Novartis Pharmaceuticals. Safety and efficacy of QAX576 in patients with idiopathic pulmonary fibrosis (IPF). https://clinicaltrials.gov/ct2/show/NCT01266135. Accessed 10 Nov 2018
Novartis Pharmaceuticals. Efficacy 2 part study of identification of keloid biomarkers and effect of QAX576 on keloid recurrence. https://clinicaltrials.gov/ct2/show/NCT00987545. Accessed 10 Nov 2018
Novartis Pharmaceuticals. QAX576 in patients with pulmonary fibrosis secondary to systemic sclerosis. https://clinicaltrials.gov/ct2/show/NCT00581997. Accessed 10 Nov 2018
Novartis Pharmaceuticals. A study to establish the efficacy of QBX258 in patients with moderate to severe asthma. https://clinicaltrials.gov/ct2/show/NCT01479595. Accessed 10 Nov 2018
O’Reilly S (2013) Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors 39:593–596
Ong CJ, Ip S, Teh SJ et al (1999) A role for T helper 2 cells in mediating skin fibrosis in tight-skin mice. Cell Immunol 196:60–68
Ong C, Wong C, Roberts CR et al (1998) Anti-IL-4 treatment prevents dermal collagen deposition in the tight-skin mouse model of scleroderma. Eur J Immunol 28:2619–2629
Oriente A, Fedarko NS, Pacocha SE et al (2000) Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 292:988–994
Panettieri RA, Sjöbring U, Péterffy A et al (2018) Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med 6:511–525
Parker JM, Glaspole IN, Lancaster LH et al (2018) A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 197:94–103
Perez OA, Berman B (2008) Cytokines and chemokines. In: Gaspari A, Tyring S (eds) Clinical and basic immunodermatology. Springer, London, pp 3–16
Postlethwaite A, Holness MA, Katai M, Raghow R (1992) Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Investig 90:1479–1485
Profyris C, Tziotzios C, Do Vale I (2012) Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics part I. The molecular basis of scar formation. J Am Acad Dermatol 66:1–10
Quirce S, Bobolea I, Domínguez-Ortega J, Barranco P (2014) Future biologic therapies in asthma. Arch Bronconeumol 50:355–361
Rabe KF, Nair P, Brusselle G et al (2018) Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 378:2475–2485
Raghu G, Richeldi L, Crestani B et al (2018) SAR156597 in idiopathic pulmonary fibrosis: a phase 2, placebo-controlled study (DRI11772). Eur Respir J 52:1801130
Rankin AL, Mumm JB, Murphy E et al (2010) IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol 184:1526–1535
Regeneron Pharmaceuticals. Dupilumab as an adjunct for subcutaneous grass immunotherapy. https://clinicaltrials.gov/ct2/show/NCT03558997. Accessed 10 Nov 2018
Regeneron Pharmaceuticals. Study to Determine the efficacy and safety of dupilumab in adult and adolescent patients with eosinophilic esophagitis (EoE). https://clinicaltrials.gov/ct2/show/NCT03633617. Accessed 10 Nov 2018
Regeneron Pharmaceuticals FDA Approves asthma indication for Dupixent® (dupilumab). https://investor.regeneron.com/news-releases/news-release-details/fda-approves-asthma-indication-dupixentr-dupilumab. Accessed 10 Nov 2018
Reinisch W, Panés J, Khurana S et al (2015) Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut 64:894–900
Renert-Yuval Y, Guttman-Yassky E (2017) The changing landscape of alopecia areata: the therapeutic paradigm. Adv Ther 34:1594–1609
Roesner LM, Zeitvogel J, Heratizadeh A (2019) Common and different roles of IL-4 and IL-13 in skin allergy and clinical implications. Curr Opin Allergy Clin Immunol 19:319–327
Rothenberg ME, Wen T, Greenberg A et al (2015) Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 135:500–507
Salmon-Ehr V, Serpier H, Nawrocki B et al (1996) Expression of interleukin-4 in scleroderma skin specimens and scleroderma fibroblast cultures. Potential role in fibrosis. Arch Dermatol 132:802–806
Salmon-Ehr V, Ramont L, Godeau G et al (2000) Implication of interleukin-4 in wound healing. Lab Investig 80:1337–1343
Sanofi. Effectiveness and safety of SAR156597 in treating diffuse systemic sclerosis. https://clinicaltrials.gov/ct2/show/NCT02921971. Accessed 10 Nov 2018
Sanofi. A controlled clinical study of dupilumab in patients with nasal polyps (SINUS-24). https://clinicaltrials.gov/ct2/show/NCT02912468. Accessed 10 Nov 2018
Sen CK, Gordillo GM, Roy S et al (2010) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 17:763–771
Shah M, Foreman DM, Ferguson MW (1994) Neutralising antibody to TGF-beta 1, 2 reduces cutaneous scarring in adult rodents. J Cell Sci 107:1137–1157
Shah M, Foreman DM, Ferguson MW (1995) Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci 108:985–1002
Shirley M (2017) Dupilumab: first global approval. Drugs 77:1115–1121
Sidgwick GP, Bayat A (2012) Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol 26:141–152
Simpson EL, Bieber T, Guttman-Yassky E et al (2016) Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 375:2335–2348
Simpson EL, Flohr C, Eichenfield LF et al (2018) Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 78:863.e11–871.e11
Swigris J, Ogura T, Scholand M et al, The RIFF Study (2018) (Cohort A): a phase II, randomized, double-blind, placebo-controlled trial of lebrikizumab as monotherapy in patients with idiopathic pulmonary fibrosis (abstract A6167). American Thoracic Society 2018 International Conference, San Diego, CA, USA, 2018
Tan H-TT, Sugita K, Akdis CA (2016) Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep 16:70
Trace AP, Enos CW, Mantel A, Harvey VM (2016) Keloids and hypertrophic scars: a spectrum of clinical challenges. Am J Clin Dermatol 17:201–223
Tredget EE, Yang L, Delehanty M et al (2006) Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interf Cytokine Res 26:179–189
Tripp CS, Cuff C, Campbell AL et al (2017) RPC4046, a novel anti-interleukin-13 antibody, blocks IL-13 binding to IL-13 α1 and α2 receptors: a randomized, double-blind, placebo-controlled, dose-escalation first-in-human study. Adv Ther 34:1364–1381
Tziotzios C, Profyris C, Sterling J (2012) Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics: part II. Strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol 66:13–24
Uitto J (1990) Fibrotic skin diseases. Arch Dermatol 126:661
Varga J, Abraham D (2007) Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Investig 117:557–567
Walmsley GG, Maan ZN, Wong VW et al (2015) Scarless wound healing: chasing the holy grail. Plast Reconstr Surg 135:907–917
Walsh GM (2018) Recent developments in the use of biologics targeting IL-5, IL-4, or IL-13 in severe refractory asthma. Expert Rev Respir Med 12:957–963
Walton KL, Johnson KE, Harrison CA (2017) Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 8:461
Weng S-Y, Wang X, Vijayan S et al (2018) IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. EBioMedicine 29:92–103
Wenzel S, Wilbraham D, Fuller R et al (2007) Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet (London, England) 370:1422–1431
Wills-Karp M, Finkelman FD (2008) Untangling the complex web of IL-4-and IL-13-mediated signaling pathways. Sci Signal 1:1–5
Wollenberg A, Howell MD, Guttman-Yassky E et al (2019) Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J Allergy Clin Immunol 143:135–141
Wynn TA (2009) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Wynn TA (2015) Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15:271–282
Wynn TA (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4:583–594
Wynn TA (2003) IL-13 effector functions. Annu Rev Immunol 21:425–456
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040
Wynn T, Barron L (2010) Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30:245–257
Zhu Z, Ding J, Ma Z et al (2016) Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Repair Regen 24:644–656
Zhu Z, Ding J, Shankowsky HA, Tredget EE (2013) The molecular mechanism of hypertrophic scar. J Cell Commun Signal 7:239–252
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award no. K23GM117309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, J.K., Austin, E., Huang, A. et al. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res 312, 81–92 (2020). https://doi.org/10.1007/s00403-019-01972-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-019-01972-3