Abstract
Aims/hypothesis
To treat obesity and related diseases, considerable effort has gone into developing strategies to convert white adipocytes into thermogenic brown-like adipocytes (‘browning’). The purpose of this study was to identify the most efficient signal control for browning.
Methods
To identify the most efficient signal control for browning, we examined rat stromal vascular fraction cells. In addition, physiological changes consequent to signal control were examined in vivo using lean and diet-induced obese (DIO) C57BL/6J mice.
Results
Combined treatment with the peroxisome proliferator-activated receptor γ (PPARγ) agonist rosiglitazone, the SMAD3 inhibitor SIS3 and the adrenergic receptor agonist noradrenaline (norepinephrine) synergistically induced Ucp1, Fgf21 and Cited1 expression, triggering brown adipogenesis. Synergistic induction of Ucp1 by the three agents was negatively regulated by forkhead box O (FOXO)3 via the inhibition of PPARγ-dependent gene transcription. Moreover, the administration of rosiglitazone, SIS3 and the selective β3 adrenergic receptor agonist CL316,243 to DIO mice reduced the amount of body-fat deposits (body weight from day 0 to 14, 12.3% reduction), concomitant with morphological changes in white adipose tissue, an increase in mitochondrial biosynthesis and a marked induction of uncoupling protein 1 (UCP1). Furthermore, administration of the three agents significantly increased serum adiponectin levels (mean 65.56 μg/ml with the three agents vs 20.79 μg/ml in control mice, p < 0.05) and improved glucose and lipid tolerance.
Conclusions/interpretation
These results suggest that the combined regulation of PPARγ, SMAD and the adrenergic receptor signalling pathway synergistically induces brown adipogenesis and may serve as an effective strategy to treat obesity and related diseases, including type 2 diabetes.
Similar content being viewed by others

Introduction
Obesity is closely related to the onset of lifestyle-related diseases such as diabetes, hypertension and cardiovascular disease, and the increase in the number of individuals affected by these diseases because of changes in dietary habits and lifestyles has become a major social problem [1]. A potentially effective strategy to prevent or treat obesity is to increase energy expenditure through uncoupling protein 1 (UCP1) by converting white adipocytes into brown-like adipocytes (‘browning’). Brown adipocytes are characterised by abundant expression of UCP1, and contain multilocular lipid droplets and many mitochondria. UCP1 decreases the proton gradient between mitochondrial membranes and dissipates energy as heat by uncoupling respiration from ATP synthesis [2, 3]. Rodent-based studies have demonstrated that interscapular brown adipose tissue (BAT) is crucial not only for cold tolerance, but also for maintaining whole-body energy homeostasis [4,5,6]. Although it had been thought that BAT diminishes with age and is less functional in adult humans [7], recent studies have shown that active brown adipocytes are present in adults [8, 9] and play a role in cold-induced thermogenesis. However, decreased activity of adipocytes associated with ageing has also been observed [10,11,12]. Thus, brown adipocytes have attracted attention as a therapeutic target for obesity and diabetes.
Brown adipocytes are classified into two types based on their differentiation lineage. Brown adipocytes in human infants and rodents are termed ‘classical brown adipocytes’ and are differentiated from myogenic factor 5 (MYF5)-positive precursor cells. In contrast, those appearing in white adipose tissue when bodies are exposed to a cold environment or adrenergic receptor agonists are termed ‘beige adipocytes’ and are derived from MYF5-negative precursor cells [13, 14]. Gene expression profiling has revealed that brown adipocytes present in adult humans are mainly composed of beige adipocytes [13, 15]. Moreover, in the last decade, molecules that regulate the differentiation and activity of brown adipocytes have been partly clarified [14]. The central regulator of these processes is peroxisome proliferator-activated receptor γ (PPARγ), with thiazolidinediones (TZDs) acting as a ligand for PPARγ to induce browning of white adipose tissue following their chronic administration in mammals or application to cultured adipocyte precursor cells [16, 17]. Another master regulator of browning, PRD1-BF1-RIZ1 homologous domain containing 16 (PRDM16), has been shown to suppress white adipocyte-selective gene expression by interacting with C-terminal-binding protein (CtBP)-1 and CtBP-2, which upon binding with PPARγ coactivator 1α (PGC1α) displaces CtBPs and induces brown adipocyte-selective gene expression, such as that of PGC1α itself [18]. In addition, TGF-β family molecules regulate the differentiation lineage of adipocytes via their receptors and downstream SMAD signalling [19, 20]. Among these, the TGF–SMAD3 axis reportedly inhibits brown adipogenesis by suppressing PGC1α expression [21]. Thus, the differentiation lineage of adipocytes is controlled by numerous transcription factors [22].
Recently, the effective control of browning has been proposed as a new approach for treating obesity and diabetes; therefore, numerous efforts have been made to explore the inducers of this process [23]. Although TZDs are known to induce thermogenic gene expression in cultured cells and mammals [16, 17], it is difficult to obtain a marked anti-obesity effect using TZDs alone because of their unwanted side effects [24]. Thus, effective means of regulating browning for the purposes of anti-obesity and diabetes treatment have not yet been established. In the present study, we investigated intracellular signals that could efficiently induce browning of rat stromal vascular fraction (SVF) cells. Furthermore, we examined the efficacy of agents that regulate browning-related signals in obesity and diabetes in mice.
Methods
Preparation of SVF cells
Minced inguinal adipose tissues from male Wistar rats (8–10 weeks old, Japan SLC, Shizuoka, Japan) were incubated in collagenase buffer (0.05% collagenase, 4% BSA in Hanks’ buffer) for 15 min at 37°C. After adding the culture medium (DMEM supplemented with 10% FBS), the cell suspension was passed through 100 μm nylon mesh and centrifuged for 5 min at 200 g. The upper layer of the suspension was removed, and the pellet was resuspended in culture medium and used as SVF. All animal experiments were approved by the Animal Care Committee of Kao Corporation and performed in accordance with the committee’s guidelines.
Cell experiments
SVF cells were cultured in DMEM (+10% FBS) on type I collagen-coated plates for 1 day (~80% confluence). Cells were then exposed to the PPARγ agonist rosiglitazone (Wako Pure Chemical, Osaka, Japan) and/or the SMAD3 inhibitor SIS3 (specific inhibitor of SMAD3; Sigma-Aldrich, St Louis, MO, USA) for the indicated time period, followed by addition of the adrenergic receptor agonist noradrenaline (norepinephrine) (Wako) for 2 h. After washing the cells with PBS, cells were used for further analyses.
RNA isolation and real-time PCR
Total RNA was isolated from cultured cells or adipose tissues using QIAzol and the RNeasy Mini Kit (Qiagen, Venlo, the Netherlands). Isolated RNA was reverse-transcribed using the High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific, Waltham, MA, USA) and real-time PCR was performed with gene-specific TaqMan probes (Thermo Fisher Scientific, see electronic supplementary material [ESM] Table 1) using a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). The expression of each gene was normalised to that of Rplp0.
Western blot analysis
Adipose tissues were homogenised in ice-cold RIPA buffer (Sigma-Aldrich) using a motor-driven pestle in a microcentrifuge tube. Cells were lysed in 1% SDS containing buffer. Supernatants were denatured at 95°C in SDS sample buffer. Equal amounts of protein were subjected to SDS-PAGE, followed by electroblotting onto a PVDF membrane. Lysates were quantified using BCA Protein Assay Kit (Thermo Fisher Scientific). Blotted membranes were probed with each primary antibody (1:1000, 4°C, 16 h; ESM Table 2), followed by a secondary antibody (1:1000) using an immunoreaction enhancer solution (Can Get Signal; TOYOBO, Osaka, Japan). Blots were visualised using the ECL prime western blotting detection system (GE Healthcare, Marlborough, MA, USA) and ImageQuant LAS 4000 (Fujifilm, Tokyo, Japan).
Immunohistochemistry
Adipose tissues were dissected and fixed in 10% formaldehyde at room temperature and embedded in paraffin. Paraffin sections were mounted on slides, deparaffinised and probed with an anti-UCP1 antibody (no. 23841; Abcam, Cambridge, UK). Visualisation was performed using the anti-rabbit EnVision+ System-HRP (Agilent Technologies, Santa Clara, CA, USA) and the Liquid DAB Substrate-Chromogen System (Agilent Technologies).
Electron microscopy
Adipose tissues were fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 mol/l cacodylate buffer, pH 7.4, at 4°C overnight, then subjected to post-fixation with 2% osmium tetroxide at 4°C for 3 h. The samples were dehydrated in a graded ethanol series (50%, 70%, 90%, 100%), infiltrated with propylene oxide and placed into a 70:30 mixture of propylene oxide and resin (Quetol-812; Nissin EM, Tokyo, Japan), after which the propylene oxide volatilised. The samples were then transferred to fresh 100% resin and polymerised at 60°C for 48 h. These blocks were ultra-thin sectioned at 70 nm using an ultramicrotome, then stained with Lead Stain Solution (18-0875; Sigma-Aldrich). The grids were observed using a transmission electron microscope (JEM-1400Plus; JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV.
RNA interference-mediated gene knockdown
Transfection of small interfering (si)RNA was performed by electroporation using the Neon Transfection System (Thermo Fisher Scientific). Rat SVF cells suspended in Buffer R (1.5 × 105 cells per electroporation) were transfected with 50 pmol target-specific Silencer siRNA (Thermo Fisher Scientific), plated onto a 24-well culture plate and cultured in DMEM (+10% FBS). Silencer siRNAs used were 4390843 (Scramble), s130429 (Smad1), s131227 (Smad2), s130318 (Smad3), s132211 (Smad4), s133234 (Smad5), s137004 (Smad8), s136654 (Foxo1), s148639 (Foxo3) and s154315 (Foxo4). At 6 h after transfection, cells were exposed to rosiglitazone and SIS3 for 4 days, followed by treatment with noradrenaline for 2 h. After washing the cells with PBS, cells were used for further analyses.
Plasmids and luciferase gene reporter assay
The 5′ flanking region of the human UCP1 gene (NCBI accession no. NM_021833.4, −4142 to +160 sequence in reference to the transcription start site) was artificially synthesised (GenScript, Piscataway, NJ, USA) and cloned into the pGL3-basic reporter vector (Promega, Madison, WI, USA). The tandem PPRE-luciferase reporter vector was purchased from Qiagen. The TM-forkhead box O (FOXO)3 encoding vector (containing a constitutively active mutant human FOXO3 in which all three Akt phosphorylation sites were mutated [triple mutation] to Ala: T32A, S253A, S315A) was artificially generated (Thermo Fisher Scientific). The reporter plasmids were transiently co-transfected with human PPARγ, human PGC1α and TM-FOXO3 expression vectors using Lipofectamine 3000 (Thermo Fisher Scientific) into HEK293 cells (ATCC, Manassas, VA, USA; free of mycoplasma contamination). After 24 h treatment with rosiglitazone, cells were lysed and the reporter activity was measured using the Dual-Luciferase Assay Kit (Promega) and a microplate reader (SH-9000Lab, HITACHI, Tokyo, Japan).
Animal experiments
All animals were housed under controlled temperatures (23–24°C) and light–dark cycle (12 h) conditions, with ad libitum feeding. The n numbers in the animal experiments were set empirically. Each n number is the minimum number of samples for which statistically significant differences can generally be detected. Experimenters were not blind to group assignment and outcome assessment, and no mouse was excluded from the experiments.
Experiment 1: in vivo assessment of treatment in wild-type mice
Male, 10-week-old, C57BL/6J Jcl mice (CLEA Japan, Tokyo, Japan) were randomly divided into four groups with similar body weight (n = 10 per group), and Alzet osmotic pumps (DURECT, Cupertino, CA, USA) containing rosiglitazone and SIS3 (delivering 5 mg [kg body weight]−1 day−1 each); CL316,243 (Sigma-Aldrich) alone (delivering 0.01 mg kg−1 day−1); rosiglitazone and SIS3 and CL316,243; or saline (154 mmol/l NaCl) were implanted s.c. on the backs of mice under anaesthesia. One week after implantation of the pumps, the mice were dissected and adipose tissues were used for further analyses.
Experiment 2: in vivo assessment of treatment in diet-induced obese mice
Male 7-week-old C57BL/6J mice were fed a high-fat diet containing 60% energy from fat (Research Diets, New Brunswick, NJ, USA) for 10 weeks to induce obesity (diet-induced obese [DIO] mice). The mice were then randomly divided into four groups (n = 8 per group) and the rectal temperature of each mouse was measured at 09:00 and 21:00 hours. Alzet osmotic pumps containing rosiglitazone and SIS3 (delivering 5 mg [kg body weight]−1 day−1 each); CL316,243 alone (delivering 0.1 mg kg−1 day−1); rosiglitazone and SIS3 and CL316,243; or saline were implanted s.c. on the backs of mice under anaesthesia. At 7 days after implantation of the pumps, mice were placed in a chamber for indirect calorimetry and allowed to acclimatise to the surroundings for 3 days. At 10 days, respiratory gas analysis was performed for 2 days as described below. At 13 days, oral glucose–lipid load testing was conducted. The rectal temperature was measured at 21:00 hours (day 12) and 09:00 hours (day 14). At 14 days, blood was collected via the postcaval vein from anaesthetised mice under non-fasting conditions, and each tissue sample was rapidly dissected. All blood analyses were conducted using commercially available kits (ESM Table 3).
Indirect calorimetry
Energy metabolism studies were performed using ARCO-2000 (ARCO System, Chiba, Japan), an MS system for respiratory analysis. \( \dot{V}{\mathrm{O}}_2 \) and \( \dot{V}{\mathrm{CO}}_2 \) were measured under ad libitum feeding conditions (high-fat diet) for 48 h (19:00 hours on day 10 to 19:00 hours on day 12). RQ was calculated from \( \dot{V}{\mathrm{O}}_2 \) and \( \dot{V}{\mathrm{CO}}_2 \) values (RQ = \( \dot{V}{\mathrm{CO}}_2 \)/\( \dot{V}{\mathrm{O}}_2 \)). Oxidation values of lipids and carbohydrates were calculated using the equations of Péronnet and Massicotte [25]. Simultaneously with calorimetry, locomotor activity was also measured using a motion analysis system (Actracer-2000; ARCO System).
Oral glucose–lipid load test
Glucose–lipid mixed emulsion was prepared by sonicating 10% corn oil solution containing 10% glucose, 1% egg lecithin and 4% fatty acid-free BSA. Following a 12 h fast, 10 ml/kg body weight glucose–lipid emulsion was administered via a gastric tube [26, 27]. Blood samples were obtained from the orbital sinus under anaesthesia with isoflurane at each time point, and blood glucose, serum triacylglycerol and NEFA concentrations were measured using a portable blood glucose meter (Accu-Chek Aviva; Roche Diagnostics, Mannheim, Germany) and the kit shown in ESM Table 3.
Statistical analysis
All measurements are expressed as mean ± SEM. Comparisons were made using Dunnett’s test. Variance analysis was followed by Tukey’s test or the Tukey–Kramer method. A p value of less than 0.05 was considered statistically significant.
Results
Synergistic induction of UCP1 with rosiglitazone, SIS3 and noradrenaline in SVF cells
In our preliminary experiment to examine the effect of agonists and antagonists of various intracellular signals on Ucp1 mRNA expression in SVF cells, we found that the combined treatment of cells with rosiglitazone, SIS3 and noradrenaline synergistically induced Ucp1 mRNA. Treatment of SVF cells with rosiglitazone and SIS3 for 7 days and noradrenaline for the final 2 h resulted in a synergistic increase in Ucp1 mRNA expression as compared with treatment with each agent alone (Fig. 1a). Use of the selective β3 adrenergic receptor agonist CL316,243 instead of noradrenaline also induced Ucp1 mRNA expression (ESM Fig. 1). To induce synergistic Ucp1 expression, treatment with rosiglitazone and SIS3 for 6–10 days followed by noradrenaline stimulation was required (Fig. 1b), with the effect of noradrenaline occurring 2 h after addition and lasting up to 8 h (Fig. 1c). Pioglitazone, a PPARγ agonist TZD compound, also showed a synergistic effect similar to that of rosiglitazone; however, a non-TZD partial agonist of PPARγ (nTZDpa) did not show a significant effect (Fig. 1d). RNA interference experiments using specific siRNA for major SMAD family molecules showed that synergistic Ucp1 mRNA expression by rosiglitazone and noradrenaline was induced in cells transfected with Smad3 and Smad4 siRNA (Fig. 1e).
Synergistic induction of Ucp1 with combined rosiglitazone, SIS3 and noradrenaline in SVF cells. (a) Rat SVF cells were untreated (control) or treated with 1 μmol/l rosiglitazone and/or 10 μmol/l SIS3 for 7 days; cells were then treated with or without 1 μmol/l noradrenaline for 2 h. *p < 0.05 vs control in the same NAd treatment group; †p < 0.05 vs Rosi in the same NAd treatment group; ‡p < 0.05 vs SIS3 in the same NAd treatment group (Tukey’s test). (b) SVF cells were untreated (control) or treated with 1 μmol/l rosiglitazone and/or 10 μmol/l SIS3 for the indicated time; cells were then treated with 1 μmol/l noradrenaline for 2 h. (c) SVF cells were treated with rosiglitazone + SIS3 for 7 days and with noradrenaline for the indicated time. (d) Comparison between three PPARγ agonists. SVF cells were treated with 1 μmol/l rosiglitazone, pioglitazone or nTZDpa in the presence or absence of SIS3, and then treated with 1 μmol/l noradrenaline for 2 h. ***p < 0.001 vs control in the SIS3- treatment group; †††p < 0.001 vs control in the SIS3+ treatment group (Dunnett’s test) (e) SVF cells were transfected with each Smad siRNA and treated with 1 μmol/l rosiglitazone and/or 10 μmol/l SIS3 for 4 days, and then with 1 μmol/l noradrenaline for 2 h. The total RNA isolated from whole cell lysate was subjected to real-time RT-PCR. The amount of Ucp1 mRNA was normalised to Rplp0 mRNA levels and is expressed as fold change of the corresponding value in the control group (left-hand untreated/Scramble control where there is more than one control). The lines drawn between the bars indicate significant differences between the groups (Dunnett’s test, ***p < 0.001). Values are means ± SEM of two (b, c) or three (a, d, e) samples. Cont, control; NAd, noradrenaline; Pio, pioglitazone; R+S, rosiglitazone + SIS3; Rosi, rosiglitazone
Expression levels of adipocyte marker genes in SVF cells treated with rosiglitazone, SIS3 and noradrenaline
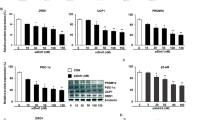
To uncover changes in cell characteristics upon treatment with the three agents, the expression of various adipocyte marker genes was analysed using real-time PCR. Prdm16 mRNA expression was not increased by any treatment (Fig. 2a), whereas the mRNA expression of PGC1α (Ppargc1a), a cofactor of PPARγ, was increased by SIS3 treatment (Fig. 2b). Cidea expression was significantly induced by rosiglitazone and SIS3, and synergistically increased by the combination treatment (Fig. 2c). Expression of Zic1, a classical brown marker, was slightly increased by SIS3 treatment (Fig. 2d), although expression of a similar marker gene, Lhx8, did not change (Fig. 2e). In contrast, the expression of Fgf21 and Cited1, which are specific marker genes of beige adipocytes, increased synergistically with the combined treatment of rosiglitazone and SIS3 (Fig. 2f,g). Similarly, the adipocyte differentiation marker genes Fabp4 and Pparg were also significantly increased by the combination treatment of rosiglitazone and SIS3 (Fig. 2k,l).
Expression level of adipocyte marker genes in cells treated with rosiglitazone, SIS3 and noradrenaline. Rat SVF cells were untreated (control) or treated with 1 μmol/l rosiglitazone and/or 10 μmol/l SIS3 for 7 days; cells were then treated with or without 1 μmol/l noradrenaline for 2 h. mRNAs of brown marker genes (a–c), classical brown marker genes (d, e), beige marker genes (f–i), a white marker gene (j) and adipocyte differentiation marker genes (k, l) were detected using real-time RT-PCR. The amount of each gene mRNA was normalised to Rplp0 mRNA levels and is expressed as a fold change of the corresponding amount in the untreated control group. Values are means ± SEM of three samples. Statistical analysis was performed for each set of four groups with or without noradrenaline. *p < 0.05 vs control in the same NAd treatment group; †p < 0.05 vs Rosi in the same NAd treatment group; ‡p < 0.05 vs SIS3 in the same NAd treatment group (Tukey’s test). Cont, control; NAd, noradrenaline; R+S, rosiglitazone + SIS3; Rosi, rosiglitazone
Decrease in FOXO3 expression by SIS3 induces expression of UCP1 and PPARγ target genes in SVF cells
The FOXO family molecules, comprising transcription factors that regulate cellular metabolism in response to insulin, are known to regulate adipocyte differentiation [28, 29]. Western blot analysis revealed that FOXO1 and FOXO3 levels were markedly decreased in cells treated with SIS3 but not with rosiglitazone (Fig. 3a). Specific siRNA-mediated knockdown of FOXO3 markedly increased Ucp1 mRNA (Fig. 3b). Furthermore, Ppargc1a, Pparg, Fgf21 and Fabp4 gene expression also increased in FOXO3-knocked down cells on combination treatment with rosiglitazone and SIS3 (Fig. 3d–g). Ucp1 gene expression was not detected in two out of three samples in each control–noradrenaline(−) group (Fig. 3c).
Effect of FOXO3 suppression on adipocyte gene expression. (a) Rat SVF cells were stimulated with 1 μmol/l rosiglitazone and/or 10 μmol/l SIS3 for 7 days. FOXO family protein levels were detected using western blotting. (b) Rat SVF cells were transfected with each Foxo siRNA and treated with 1 μmol/l rosiglitazone and 10 μmol/l SIS3 for 4 days, and then with 1 μmol/l noradrenaline for 2 h. The total RNA isolated from whole cell lysate was subjected to real-time RT-PCR. The amount of Ucp1 was normalised to Rplp0 mRNA levels and is expressed as a fold change of the corresponding amount in the Scramble group. *p < 0.05 vs Scramble siRNA transfected group; †p < 0.05 vs Foxo1 siRNA transfected group; ‡p < 0.05 vs Foxo3 siRNA transfected group (Tukey’s test). (c–g) Scramble (white bars) or Foxo3-specific (grey bars) siRNA transfected cells were untreated (control) or treated with 1 μmol/l rosiglitazone and 10 μmol/l SIS3 for 4 days; cells were then treated with or without 1 μmol/l noradrenaline for 2 h. The amount of each marker gene mRNA was normalised to Rplp0 mRNA levels and is expressed as a fold change of the corresponding amount in the untreated, Scramble siRNA transfected control group. *p < 0.05 vs control in the same siRNA transfection group; †p < 0.05 vs Scramble siRNA transfected group in the same treatment (Tukey’s test). Values are means ± SEM of three samples. Cont, control; NAd, noradrenaline; R+S, rosiglitazone + SIS3; Rosi, rosiglitazone
Suppression of PPARγ/PGC1α-dependent transcriptional activity by FOXO3
We evaluated the effect of FOXO3 on PPARγ-dependent transcriptional activity in HEK293 cells. Treatment of cells co-transfected with PPARγ and PGC1α expression vectors and a PPRE-luciferase vector with rosiglitazone markedly increased PPAR-dependent luciferase activity (Fig. 4a), whereas this was significantly attenuated by co-expression of a constitutively active FOXO3 generated by triple mutation (TM-FOXO3). Similarly, the activity of the human UCP1 promoter was increased by rosiglitazone treatment but suppressed by co-expression of TM-FOXO3 (Fig. 4b).
PPARγ activation induced by rosiglitazone and PGC1α is suppressed by the active form of FOXO3. HEK293 cells were transfected with PPRE (a) or UCP1 promoter (b) reporter plasmids. Concomitantly, PPARγ, PGC1α and TM-FOXO3 expression plasmids were co-transfected. The cells were treated with 1 μmol/l rosiglitazone for 24 h, and firefly luciferase activity was measured. Firefly luciferase activity was normalised to Renilla luciferase as relative luciferase activity and is expressed as a fold change of the corresponding amount in the control (no gene transfected, [a] Rosi+, [b] Rosi−) group. Values are means ± SEM of three samples. *p < 0.05 vs no vector transfected control in the same Rosi treatment group; †p < 0.05 vs PPARγ transfected in the same Rosi treatment group; ‡p < 0.05 vs PPARγ and PGC1α transfected in the same Rosi treatment group (Tukey’s test). Luc, luciferase; Rosi, rosiglitazone
Induction of UCP1 by a combination of rosiglitazone, SIS3 and CL316,243 in C57BL/6J mice
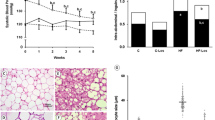
Next, we examined the synergistic induction of UCP1 expression by the three-agent combination in in vivo experiments. In these experiments, CL316,243, a synthetic β3 adrenergic receptor agonist, was used instead of noradrenaline for pharmacological reasons [30]. An osmotic pump was placed in the back of mice s.c., and rosiglitazone, SIS3 and CL316,243 were chronically administered for 1 week. Expression analyses for each adipose tissue revealed a marked increase in Ucp1 mRNA and protein expression in inguinal white adipose tissue (iWAT) with administration of the three agents (Fig. 5a, d, e). Similarly, expression of brown and beige marker genes was increased synergistically with administration of the three agents (ESM Fig. 2). The mRNA expression of Nfe2l1, a newly described thermogenic adaptation regulator, was significantly induced by the three agents (ESM Fig. 3). In transmission electron microscopy analysis of iWAT, miniaturisation of lipid droplets and hyperplasia of mitochondria by the three agents were observed (Fig. 5f).
Induction of UCP1 by a combination of rosiglitazone, SIS3 and CL316,243 in C57BL/6J mice. The mice were administered saline (control) or rosiglitazone and SIS3 (each 5 mg [kg body weight]–1 day−1), with or without CL316,243 (0.01 mg [kg body weight]−1 day−1); all treatments were s.c. using an osmotic pump for 7 days. Total RNA isolated from each adipose tissue, namely iWAT (a), epididymal white adipose tissue (b) and interscapular BAT (c), was subjected to real-time RT-PCR. The amount of Ucp1 mRNA was normalised to the Rplp0 mRNA levels and is expressed as fold change of the corresponding amount in iWAT of the control without CL316,243 group. Values are means ± SEM of nine or ten (b, rosiglitazone + SIS3 group) mice (a–c). *p < 0.05 vs control without CL316,243 treatment group; †p < 0.05 vs R+S without CL316,243 treatment group; ‡p < 0.05 vs control with CL316,243 treatment group (Tukey’s test). (d) iWAT lysates were prepared and subjected to western blot analysis using specific antibodies for UCP1 and α-tubulin. (e) Representative UCP1 immunostaining in iWAT from each group. Scale bar, 200 μm. (f) iWAT sections were subjected to transmission electron microscopy. Scale bar, 10 μm. Cont, control; eWAT, epididymal white adipose tissue; iBAT, interscapular BAT; R+S, rosiglitazone + SIS3
Effects of combined administration of rosiglitazone, SIS3 and CL316,243 in DIO mice
We further evaluated the effect of browning of white adipose by the three agents in DIO mice. Chronic administration of the three agents using a subcutaneous osmotic pump for 2 weeks caused synergistic UCP1 expression in obese mice (ESM Fig. 4). At 13 days after starting administration, an oral glucose–lipid tolerance test was performed. The blood glucose level increased markedly 15 min after oral administration of the emulsion in all groups, but was significantly lower in the all three-agent-treated (combination treatment) group compared with the control group (Fig. 6a) (AUC [mmol/l × min]: control 1976; rosiglitazone + SIS3 1737; CL316,243 1809; combination treatment 1523). Blood insulin levels were lower in the CL316,243 and combination treatment group than in the control group at 15 and 30 min after administration (Fig. 6b) (AUC [pmol/l × min]: control 104,581; rosiglitazone + SIS3 52,081; CL316,243 45,149; combination treatment 44,798; significantly different between control vs CL316,243, control vs combination treatment; Tukey’s test, p < 0.05). Blood triacylglycerol (Fig. 6c) (AUC [mmol/l × min]: control 206.7; rosiglitazone + SIS3 122.0; CL316,243 175.9; combination treatment 118.6; Significantly different control vs rosiglitazone+SIS3, control vs combination treatment group) and NEFA levels (Fig. 6d) (AUC [mEq/l × min]: control 126.7; rosiglitazone + SIS3 84.8; CL316,243 109.4; combination treatment 76.3; Significantly different control vs rosiglitazone+SIS3, control vs combination treatment, CL316,243 vs combination treatment group) were lower in the rosiglitazone + SIS3 and combination treatment groups than in the control group.
Effects of combined administration of rosiglitazone, SIS3 and CL316,243 on the postprandial response of serum components in obese mice. DIO mice were administered rosiglitazone, SIS3 and/or CL316,243 s.c. using an osmotic pump. At 13 days after administration of the compounds, an oral glucose–lipid load test was conducted. Time-dependent changes in blood glucose (a), serum insulin (b), triacylglycerol (c) and NEFA (d) after glucose–lipid loading were determined. Values are means ± SEM of eight mice. *p < 0.05 vs control at each time point (Tukey–Kramer method)
The body weight of mice at the end of 2 weeks administration was significantly lower in the combination treatment group than control group (Table 1). The body weight of the combination treatment group decreased by 12.3% compared with before administration. Although it decreased slightly in the rosiglitazone + SIS3 and CL316,243 groups, the difference was not significant in comparison with the control group. The tissue weight of each type of white adipose tissue also decreased in the combination treatment group. In comparison, the weights of BAT, liver and muscle tissues did not change. The core body temperature was significantly higher in the combination treatment group than in the control group during both the day and night. Blood insulin and triacylglycerol levels were significantly lower in the combination treatment group, whereas the adiponectin level increased more than threefold compared with that in the control group.
\( \dot{V}{\mathrm{O}}_2 \), measured by indirect calorimetry after a 10-day administration of the three agents, showed a non-significant increase of about 14% and RQ showed a non-significant decrease, compared with the control group (ESM Fig. 5). During this period, no difference was found in the locomotor activity between groups (data not shown).
Discussion
In the present study, we revealed that the combined treatment of SVF cells with a PPARγ agonist, SMAD3 inhibitor and adrenergic receptor agonist dramatically induced browning and UCP1 expression, and that these effects were negatively regulated by FOXO3. Furthermore, we have shown that combined administration of the three agents to obese mice effectively increased heat production, reduced body fat and improved glucose tolerance, which accompanied the induction of UCP1 expression in white adipose tissue.
Gene profiling analysis revealed that the expression of beige marker genes, including Fgf21 and Cited1, was induced by the three-agent treatment, whereas the expression of classical brown marker genes such as Zic1 and Lhx8 did not change, suggesting that the browning of adipose tissue by the three agents likely occurred owing to the induction of beige adipogenesis. Similar to previous results observed for rosiglitazone and noradrenaline treatment [16], treatment with rosiglitazone and SIS3 for at least 4 days, along with subsequent short-term treatment with noradrenaline, was necessary to obtain the synergistic induction of Ucp1 expression by the three agents. In particular, long-term treatment with rosiglitazone increases the cellular amount of PRDM16 by extending the protein’s half-life [31], suggesting a contribution of PRDM16 in the three-agent-induced browning and Ucp1 expression. Moreover, our results further show that inhibiting SMAD3 signalling in combination with rosiglitazone and noradrenaline is highly effective in inducing browning. SMAD3, which is a target molecule of SIS3, is activated by TGF-β and functions as a transcription regulation factor by binding to SMAD-binding elements [32]. Considering the previous finding that SMAD3 suppresses the expression of PGC1α, a coactivator of PPARγ, by binding to its promoter region [21], SIS3 treatment may upregulate PGC1α by inhibiting SMAD3 and thereby increasing PPARγ-dependent UCP1 expression.
In the present study, multiple lines of evidence suggest that FOXO3 functions as a negative regulator of PPARγ and browning. A predicted mechanism of action is that FOXO3 inhibits the activity of PPARγ by direct protein–protein interaction. In particular, the previous demonstration by coimmunoprecipitation analysis that FOXO1, another member of the FOXO family, directly binds to PPARγ and suppresses the transcriptional activity of PPARγ in adipocytes [33] suggests the possibility that FOXO3 inhibited PPARγ function via a similar mechanism. Notably, however, the inhibition of FOXO3 had a stronger effect on UCP1 expression than did FOXO1 in RNA interference experiments. Although FOXO1 and FOXO3 share sequence motifs and regulate similar target genes [28], each type of FOXO is considered to control diverse biological responses depending on its unique post-translational modifications, cofactor selection and expression level, as indicated by the different phenotype of each FOXO subfamily member-deficient animal model [34, 35], suggesting the possibility that FOXO3 plays a different role in browning than FOXO1. The protein–protein interaction between FOXO3 and PPARγ, as well as the detailed regulation mechanisms of UCP1 expression by the different FOXO proteins, remain to be determined.
Chronic administration of the three agents strongly induced UCP1 expression in mice, especially in subcutaneous adipose tissue. In particular, in the subcutaneous fat of mice administered the three agents, small, multilocular lipid droplets characteristic of BAT were observed, suggesting that the combination treatment induces thermogenic gene expression as well as adipose tissue remodelling. Electron microscopy analysis of the subcutaneous fat showed the presence of numerous developed mitochondria in combination treatment mice. As PGC1α functions as a cofactor of nuclear respiratory factor-1/2 and induces the expression of mitochondriogenesis-related genes [36], the increased expression of PGC1α by SMAD3 inhibition might lead to PGC1α-dependent mitochondriogenesis. Alternatively, recent reports indicate that mitophagy, which is a mitochondrial-specific autophagy mechanism, controls brown adipocyte mitochondrial numbers [37]. The findings that FOXO3 decreases the number of mitochondria by activating mitophagy via PINK1 gene expression [38] and that FOXO3 expression is lower in mitochondrial-rich BAT than in white adipose tissue [39] may indicate that FOXO3 contributes to three-agent treatment-mediated browning by regulating the number of mitochondria.
The induction of browning by the three-agent treatment resulted in a significant decrease in body weight and fat mass in obese mice, which was attributable to increased thermogenesis via UCP1 expression and a possible increase in energy expenditure. In addition, administration of the three agents markedly increased serum adiponectin and decreased insulin levels, and improved glucose and lipid tolerance. The increased clearance of blood triacylglycerol by the three agents, which is similar to the results following cold exposure [40], suggests increased activity of beige/brown adipocytes and their possible contribution to the metabolic improvement. Furthermore, increased expression of Nfe2l1, which regulates metabolic and thermogenic function in adaptive thermogenesis [41], may also support the contribution of three-agent-induced browning towards the metabolic improvement in obese mice.
Adiponectin, an adipocytokine secreted from adipose tissue, exhibits many valuable functions including improvement of insulin sensitivity and anti-atherogenic effects [42]. Therefore, the induction of browning by the three agents may be expected to exert an improving and preventive effect towards diabetes, hypertension and cardiovascular diseases by increasing adiponectin production, in addition to the body-fat-reducing effect.
Although we did not observe negative effects in the three-agent group, the safety of systemic SMAD3 inhibition over a long period needs to be scrutinised to fully clarify the efficacy of this method [43].
In summary, we have revealed that the combined regulation of three signals could be useful for inducing UCP1 expression and browning in subcutaneous adipose tissue, thereby leading to reductions in obesity and improvements in various metabolic variables. Furthermore, we identified FOXO3 as a new regulatory factor in browning and showed that FOXO3 suppresses UCP1 expression by negatively regulating PPARγ. Our findings suggest that the synergistic induction of browning by modulating these three signalling pathways may constitute a new and valuable strategy for the treatment of obesity and type 2 diabetes.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAT:
-
Brown adipose tissue
- CtBP:
-
C-terminal-binding protein
- DIO:
-
Diet-induced obese
- FOXO:
-
Forkhead box O
- iWAT:
-
Inguinal white adipose tissue
- MYF 5 :
-
Myogenic factor 5
- nTZDpa :
-
Non-thiazolidinedione partial agonist of peroxisome proliferator-activated receptor γ
- PGC1α:
-
Peroxisome proliferator-activated receptor γ coactivator 1α
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- PRDM16:
-
PRD1-BF1-RIZ1 homologous domain containing 16
- siRNA:
-
Small interfering RNA
- SIS3:
-
Specific inhibitor of SMAD3
- SVF:
-
Stromal vascular fraction
- TZD:
-
Thiazolidinedione
- UCP1:
-
Uncoupling protein 1
References
Bray GA, Bellanger T (2006) Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 29(1):109–117. https://doi.org/10.1385/ENDO:29:1:109
Cedikova M, Kripnerová M, Dvorakova J, et al (2016) Mitochondria in white, brown, and beige adipocytes. Stem Cells Int 6067349. https://doi.org/10.1155/2016/6067349
Klingenberg M (1999) Uncoupling protein—a useful energy dissipator. J Bioenerg Biomembr 31(5):419–430. https://doi.org/10.1023/A:1005440221914
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359. https://doi.org/10.1152/physrev.00015.2003
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9(2):203–209. https://doi.org/10.1016/j.cmet.2008.12.014
Stanford KI, Middelbeek RJ, Townsend KL et al (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123(1):215–223. https://doi.org/10.1172/JCI62308
Heaton JM (1972) The distribution of brown adipose tissue in the human. J Anat 112(pt 1):35–39
Saito M, Okamatsu-Ogura Y, Matsushita M et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58(7):1526–1531. https://doi.org/10.2337/db09-0530
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360(15):1518–1525. https://doi.org/10.1056/NEJMoa0808949
Yoneshiro T, Aita S, Matsushita M et al (2011) Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19(9):1755–1760. https://doi.org/10.1038/oby.2011.125
Yoneshiro T, Aita S, Matsushita M et al (2011) Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 19(1):13–16. https://doi.org/10.1038/oby.2010.105
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360(15):1500–1508. https://doi.org/10.1056/NEJMoa0808718
Wu J, Boström P, Sparks LM et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376. https://doi.org/10.1016/j.cell.2012.05.016
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263. https://doi.org/10.1038/nm.3361
Sharp LZ, Shinoda K, Ohno H et al (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7(11):e49452. https://doi.org/10.1371/journal.pone.0049452
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J (2010) Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285(10):7153–7164. https://doi.org/10.1074/jbc.M109.053942
Rong JX, Qiu Y, Hansen MK et al (2007) Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56(7):1751–1760. https://doi.org/10.2337/db06-1135
Kajimura S, Seale P, Tomaru T et al (2008) Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 22(10):1397–1409. https://doi.org/10.1101/gad.1666108
Yoshida H, Kanamori Y, Asano H et al (2013) Regulation of brown adipogenesis by the Tgf-β family: involvement of Srebp1c in Tgf-β- and activin-induced inhibition of adipogenesis. Biochim Biophys Acta 1830(11):5027–5035. https://doi.org/10.1016/j.bbagen.2013.06.036
Tseng YH, Kokkotou E, Schulz TJ et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454(7207):1000–1004. https://doi.org/10.1038/nature07221
Yadav H, Quijano C, Kamaraju AK et al (2011) Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14(1):67–79. https://doi.org/10.1016/j.cmet.2011.04.013
Park JH, Hur W, Lee SB (2015) Intricate transcriptional networks of classical brown and beige fat cells. Front Endocrinol (Lausanne) 6:124. https://doi.org/10.3389/fendo.2015.00124
Song NJ, Chang SH, Li DY, Villanueva CJ, Park KW (2017) Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules. Exp Mol Med 49(7):e353. https://doi.org/10.1038/emm.2017.70
Wilding J (2006) Thiazolidinediones, insulin resistance and obesity: finding a balance. Int J Clin Pract 60(10):1272–1280. https://doi.org/10.1111/j.1742-1241.2006.01128.x
Péronnet F, Massicotte D (1991) Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16(1):23–29
Shimotoyodome A, Fukuoka D, Suzuki J et al (2009) Coingestion of acylglycerols differentially affects glucose-induced insulin secretion via glucose-dependent insulinotropic polypeptide in C57BL/6J mice. Endocrinology 150(5):2118–2126. https://doi.org/10.1210/en.2008-1162
Murase T, Yokoi Y, Misawa K et al (2012) Coffee polyphenols modulate whole-body substrate oxidation and suppress postprandial hyperglycaemia, hyperinsulinaemia and hyperlipidaemia. Br J Nutr 107(12):1757–1765. https://doi.org/10.1017/S0007114511005083
Onuma H, Vender Kooi BT, Boustead JN, Oeser JK, O’Brien RM (2006) Correlation between FOXO1 (FKHR) and FOXO3a (FKHRL1) binding and the inhibition of basal glucose-6-phosphatase catalytic subunit gene transcription by insulin. Mol Endocrinol 20(11):2831–2847. https://doi.org/10.1210/me.2006-0085
Munekata K, Sakamoto K (2009) Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell Dev Biol Anim 45(10):642–651. https://doi.org/10.1007/s11626-009-9230-5
MacPherson RE, Castellani L, Beaudoin MS, Wright DC (2014) Evidence for fatty acids mediating CL 316,243-induced reductions in blood glucose in mice. Am J Physiol Endocrinol Metab 307(7):E563–E570. https://doi.org/10.1152/ajpendo.00287.2014
Ohno H, Shinoda K, Spiegelman BM, Kajimura S (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15(3):395–404. https://doi.org/10.1016/j.cmet.2012.01.019
Feng XH, Derynck R (2005) Specificity and versatility in TGF-β signaling through SMADs. Annu Rev Cell Dev Biol 21(1):659–693. https://doi.org/10.1146/annurev.cellbio.21.022404.142018
Fan W, Imamura T, Sonoda N et al (2009) FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 284(18):12188–12197. https://doi.org/10.1074/jbc.M808915200
Tzivion G, Dobson M, Ramakrishnan G (2011) FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 1813(11):1938–1945. https://doi.org/10.1016/j.bbamcr.2011.06.002
Nakae J, Oki M, Cao Y (2008) The FoxO transcription factors and metabolic regulation. FEBS Lett 582(1):54–67. https://doi.org/10.1016/j.febslet.2007.11.025
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res 79(2):208–217. https://doi.org/10.1093/cvr/cvn098
Altshuler-Keylin S, Shinoda K, Hasegawa Y et al (2016) Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab 24(3):402–419. https://doi.org/10.1016/j.cmet.2016.08.002
Webb AE, Brunet A (2014) FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci 39(4):159–169. https://doi.org/10.1016/j.tibs.2014.02.003
Furuyama T, Nakazawa T, Nakano I, Mori N (2000) Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J 349(pt 2):629–634. https://doi.org/10.1042/bj3490629
Bartelt A, Bruns OT, Reimer R et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17(2):200–205. https://doi.org/10.1038/nm.2297
Bartelt A, Widenmaier SB, Schlein C et al (2018) Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat Med 24(3):292–303. https://doi.org/10.1038/nm.4481
Ruan H, Dong LQ (2016) Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol 8(2):101–109. https://doi.org/10.1093/jmcb/mjw014
Zhu Y, Richardson JA, Parada LF, Graff JM (1998) Smad3 mutant mice develop metastatic colorectal cancer. Cell 94(6):703–714. https://doi.org/10.1016/S0092-8674(00)81730-4
Acknowledgements
We thank our colleagues in the Biological Science Laboratories, Kao Corporation, for the helpful discussions.
Funding
This work was supported financially by Kao Corporation. The study sponsor was not involved in the design of the study; the collection, analysis or interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
TMu conceived and designed the research. TMa and SK performed experiments. TMu, SK and TMa wrote the manuscript and had final approval of the version to be published. TMu is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 359 kb)
Rights and permissions
About this article
Cite this article
Matsumoto, T., Kiuchi, S. & Murase, T. Synergistic activation of thermogenic adipocytes by a combination of PPARγ activation, SMAD3 inhibition and adrenergic receptor activation ameliorates metabolic abnormalities in rodents. Diabetologia 62, 1915–1927 (2019). https://doi.org/10.1007/s00125-019-4938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-4938-6