Abstract
Purpose
Vitrification is a well-accepted fertility preservation procedure for cryopreservation of oocytes and embryos but little is known regarding ovarian tissue, for which slow freezing is the current convention. The aim of the present study was to assess the efficiency of non-equilibrium vitrification compared to conventional slow freezing for ovarian cortex cryopreservation.
Methods
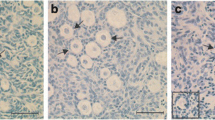
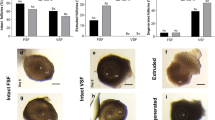
Using prepubertal sheep ovaries, the capacity of the tissue to sustain folliculogenesis following cryopreservation and in vitro culture was evaluated. Ovarian cortex fragments were cultured in wells for 9 days, immediately or after cryopreservation by conventional slow freezing or non-equilibrium vitrification in straws. During culture, follicular populations within cortex were evaluated by histology and immunohistochemistry for PCNA and TUNEL. Steroidogenic activity of the tissue was monitored by assay for progesterone and estradiol in spent media.
Results
No significant differences in follicle morphology, PCNA, or TUNEL labeling were observed between cryopreservation methods at the initiation of culture. Similar decreases in the proportion of primordial follicle population, and increases in the proportion of growing follicles, were observed following culture of fresh or cryopreserved ovarian tissue regardless of cryopreservation method. At the end of culture, PCNA and TUNEL-positive follicles were not statistically altered by slow freezing or vitrification in comparison to fresh cultured fragments.
Conclusions
Overall, for both cryopreservation methods, the cryopreserved tissue showed equal capacity to fresh tissue for supporting basal folliculogenesis in vitro. Taken together, these data confirm that both non-equilibrium vitrification and slow-freezing methods are both efficient for the cryopreservation of sheep ovarian cortex fragments.






Similar content being viewed by others
References
Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11.
Wallace WHB, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS ONE [Internet]. 2010 [cited 2017 Apr 22];5. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2811725/
Donnez J, Dolmans M-M. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49.
De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302–10.
Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at-196°C. Hum Reprod. 1994;9:597–603.
Donnez J, Dolmans M, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–70.
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–36.
Anderson RA, Wallace WHB. Fertility preservation in girls and young women. Clin Endocrinol. 2011;75:409–19.
Gavish Z, Spector I, Peer G, Schlatt S, Wistuba J, Roness H, et al. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J Assist Reprod Genet. 2018;35:61–9.
Gavish Z, Peer G, Hadassa R, Yoram C, Meirow D. Follicle activation and “burn-out” contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod. 2014;29:989–96.
Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95:695–701.
Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod BioMed Online. 2009;18:568–77.
Migishima F, Suzukimigishima R, Quintero R, Yokoyama M, Behr B. Successful pregnancies after transplantation of frozen–thawed mouse ovaries into chimeric mice that received lethal-dose radiation. Fertil Steril. 2006;86:1080–7.
Bordes A, Lornage J, Demirci B, Franck M, Courbiere B, Guerin JF, et al. Normal gestations and live births after orthotopic autograft of vitrified–warmed hemi-ovaries into ewes. Hum Reprod. 2005;20:2745–8.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15.
Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 37:1092–101.
Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–83.
Amorim CA, Curaba M, Van Langendonckt A, Dolmans M-M, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;23:160–86.
Amorim CA, Dolmans M-M, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–1298.e2.
Zhou X-H, Wu Y-J, Shi J, Xia Y, Zheng S-S. Cryopreservation of human ovarian tissue: comparison of novel direct cover vitrification and conventional vitrification. Cryobiology. 2010;60:101–5.
Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep [Internet]. 2017 [cited 2018 Jul 7];7. Available from: http://www.nature.com/articles/s41598-017-09005-7
Dalman A, Deheshkar Gooneh Farahani NS, Totonchi M, Pirjani R, Ebrahimi B, Rezazadeh Valojerdi M. Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: an evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. Cryobiology. 2017;79:29–36.
Fabbri R, Vicenti R, Macciocca M, Martino NA, Dell’Aquila ME, Pasquinelli G, et al. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod. 2016;31:1838–49.
Donfack NJ, Alves KA, Alves BG, Rocha RMP, Bruno JB, Lima LF, et al. In vivo and in vitro strategies to support caprine preantral follicle development after ovarian tissue vitrification. Reprod Fertil Dev. 2018;30:1055–65.
Nikiforov D, Russo V, Nardinocchi D, Bernabò N, Mattioli M, Barboni B. Innovative multi-protectoral approach increases survival rate after vitrification of ovarian tissue and isolated follicles with improved results in comparison with conventional method. J Ovarian Res. 2018;11:65.
Canépa S, Lainé A-L, Bluteau A, Fagu C, Flon C, Monniaux D. Validation d’une méthode immunoenzymatique pour le dosage de la progestérone dans le plasma des ovins et des bovins. Cah Techn Inra. 2008;64:19–30.
McDonald JH. Introduction - Handbook of Biological Statistics [Internet]. Handb Biol Stat. 3rd Ed. 2014. Available from: http://www.biostathandbook.com/
Bertoldo MJ, Bernard J, Duffard N, Tsikis G, Alves S, Calais L, et al. Inhibitors of c-Jun phosphorylation impede ovine primordial follicle activation. Mol Hum Reprod. 2016;22:338–49.
Bertoldo MJ, Walters KA, Ledger WL, Gilchrist RB, Mermillod P, Locatelli Y. In-vitro regulation of primordial follicle activation: challenges for fertility preservation strategies. Reprod BioMed Online. 2018;36:491–9.
Donfack NJ, Alves KA, Alves BG, Rocha RMP, Bruno JB, Lobo CH, et al. Xenotransplantation of goat ovary as an alternative to analyse follicles after vitrification. Reprod Domest Anim. 2019;54:216–24.
Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;55:261–8.
Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138:319–27.
Kim JY. Control of ovarian primordial follicle activation. Clin Exp Reprod Med. 2012;39:10–4.
Carvalho AA, Faustino LR, Silva CMG, Castro SV, Luz HKM, Rossetto R, et al. Influence of vitrification techniques and solutions on the morphology and survival of preantral follicles after in vitro culture of caprine ovarian tissue. Theriogenology. 2011;76:933–41.
Bandeira FT, Carvalho AA, Castro SV, Lima LF, Viana DA, Evangelista J, et al. Two methods of vitrification followed by in vitro culture of the ovine ovary: evaluation of the follicular development and ovarian extracellular matrix. Reprod Domest Anim. 2015;50:177–85.
Adib S, Valojerdi MR, Alikhani M. Evaluation of apoptotic markers and tissue histology indicate a slight advantage of slow freezing method over vitrification for sheep ovarian tissues. Cryo-Letters. 2018;39:313–21.
Vatanparast M, Khalili MA, Yari N, Omidi M, Mohsenzadeh M. Evaluation of sheep ovarian tissue cryopreservation with slow freezing or vitrification after chick embryo chorioallantoic membrane transplantation. Cryobiology. 2018;81:178–84.
Bertoldo MJ, Duffard N, Bernard J, Frapsauce C, Calais L, Rico C, et al. Effects of bone morphogenetic protein 4 (BMP4) supplementation during culture of the sheep ovarian cortex. Anim Reprod Sci. 2014;149:124–34.
Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod Oxf Engl. 1997;12:1993–2001.
Silva JRV, van den Hurk R, de Matos MHT, dos Santos RR, Pessoa C, de Moraes MO, et al. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–704.
Merdassi G, Mazoyer C, Guerin JF, Saad A, Salle B, Lornage J. Examination of viability and quality of ovarian tissue after cryopreservation using simple laboratory methods in ewe. Reprod Biol Endocrinol. 2011;9:78.
Faustino LR, Carvalho AA, Silva CMG, Rossetto R, Lopes CAP, van Tilburg MF, et al. Assessment of DNA damage in goat preantral follicles after vitrification of the ovarian cortex. Reprod Fertil Dev. 2015;27:440–8.
Tingen CM, Bristol-Gould SK, Kiesewetter SE, Wellington JT, Shea L, Woodruff TK. Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways1. Biol Reprod. 2009;81:16–25.
Isachenko E, Isachenko V, Rahimi G, Nawroth F. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Eur J Obstet Gynecol Reprod Biol. 2003;108:186–93.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806.
Liebenthron J, Köster M, Drengner C, Reinsberg J, van der Ven H, Montag M. The impact of culture conditions on early follicle recruitment and growth from human ovarian cortex biopsies in vitro. Fertil Steril. 2013;100:483–491.e5.
Asadi E, Najafi A, Moeini A, Pirjani R, Hassanzadeh G, Mikaeili S, et al. Ovarian tissue culture in the presence of VEGF and fetuin stimulates follicle growth and steroidogenesis. J Endocrinol. 2017;232:205–19.
Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–86.
Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40.
Acknowledgments
The authors wish to thank Thierry Delpuech for the collection of ovaries at slaughterhouse, Dr. Charlène Rico for the help with setup of culture conditions, and Corinne Laclie and Anne-Lyse Lainé from Laboratoire Phénotypage-Endocrinologie for the P4 assay.
Funding
Dr. Michael J. Bertoldo, Laure Calais, and the laboratories involved in the present study were supported by a grant from “Région Centre” (CRYOVAIRE, Grant number no. 320000268).
Author information
Authors and Affiliations
Contributions
YL designed and performed the experiments and analysis, wrote the manuscript, and secured the funding. LC performed the experiments and analysis and contributed to first draft of the manuscript. ND performed the experiments and LL performed the assay for estradiol. DM, PP, and PM contributed to experimental design and revised the manuscript. MJB performed the experiments and analysis, wrote, and revised the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Locatelli, Y., Calais, L., Duffard, N. et al. In vitro survival of follicles in prepubertal ewe ovarian cortex cryopreserved by slow freezing or non-equilibrium vitrification. J Assist Reprod Genet 36, 1823–1835 (2019). https://doi.org/10.1007/s10815-019-01532-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01532-8