Abstract
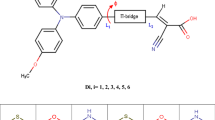
We report a computational study of a series of organic dyes built with triphenylamine (TPA) as an electron donor group. We designed a set of six dyes called (TPA-n, where n = 0–5). In order to enhance the electron-injection process, the electron-donor effect of some specific substituent was studied. Thus, we gave insights into the rational design of organic TPA-based chromophores for use in dye-sensitized solar cells (DSSCs). In addition, we report the HOMO, LUMO, the calculated excited state oxidized potential Edye*(eV) and the free energy change for electron-injection ΔGinject(eV), and the UV-visible absorption bands for TPA-n dyes by a time-dependent density functional theory (TDDFT) procedure at the B3LYP and CAM-B3LYP levels with solvent effect. The results demonstrate that the introduction of the electron-acceptor groups produces an intramolecular charge transfer showing a shift of the absorption wavelengths of TPA-n under studies.

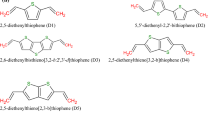
Several organic dyes TPA-n with different donors and acceptors are modeled. A strong conjugation acrros the donor and anchoring groips (TPA-n) bas been studied. Candidate TPA-3 shows a promising results.







Similar content being viewed by others
References
Singh G (2013) Energy 53:1–350
Grätzel M (2009) Acc Chem Res 42:1788–1798
Urbani M, Grätzel M, Nazeeruddin MK, Torres T (2014) Chem Rev 114:12330–12396
Pastore M, Fantacci S, De Angelis F (2013) J Phys Chem C 117:3685–3700
Casanova D, Rotzinger FP, Grätzel M (2010) J Chem Theory Comput 6:1219–1227
De Angelis F, Fantacci S, Mosconi E, Nazeeruddin MK, Grätzel M (2011) J Phys Chem C 115:8825–8831
Funaki T, Funakoshi H, Kitao O, Onozawa-Komatsuzaki N, Kasuga K, Sayama K, Sugihara H (2012) Angew Chem Int Ed 51:7528–7531
Ning Z, Tian H (2009) Chem Commun 45:5483–5495
Yum J-H, Walter P, Huber S, Rentsch D, Geiger T, Nuesch F, De Angelis F, Grätzel M, Nazeeruddin MK (2007) J Am Chem Soc 129:10320–10321
Kwon T-H, Armel V, Nattestad A, MacFarlane DR, Bach U, Lind SJ, Gordon KC, Tang W, Jones DJ, Holmes AB (2011) J Org Chem 76:4088–4093
Guo K, Yan K, Lu X, Qiu Y, Liu Z, Sun J, Yan F, Guo W, Yang S (2012) Org Lett 14:2214–2217
Imahori H, Umeyama T, Ito S (2009) Acc Chem Res 42:1809–1818
Yella A, Lee HW, Tsea HN, Yi CY, Chandirian AK, Nazeeruddin MK, Diau EWG, Yeh CY, Zakeeruddin SM, Grätzet M (2011) Science 334:629–634
Wenger S, Bouit P-A, Chen Q, Teuscher JI, Censo DD, Humphry-Baker R, Moser J-E, Delgado JL, Marín N, Zakeeruddin SM, Grätzel M (2010) J Am Chem Soc 132:5164–5169
Zhang J, Li H-B, Sun S-L, Geng Y, Wu Y, Su Z-M (2012) J Mater Chem 22:568–576
Mishra A, Fischer MKR, Bäuerle P (2009) Angew Chem Int Ed 48:2474–2499
Ooyama Y, Harima Y (2012) Chem Phys Chem 13:4032–4080
Kanaparthi RK, Kandhadi J, Giribabu L (2012) Tetrahedron 68:8383–8393
Diebolt U (2003) Surf Sci Rep 48:53–229
Macwan DP, Dave PN, Chaturvedi S (2011) J Mater Sci 46:3669–3686
Kullgren J, Huy HA, Aradi B, Frauenhein T, Deák P (2014) Status Solidi RRL 8:566–570
Valencia S, Marín JM (2010) Restrepogo. Open Mater Sci J 4:9–14
Buraidah MH, Teo LP, Yusuf SNF, Noor MM, Kufian MZ, Careem MA, Majid SR, Taha RM, Arof AK (2011) Int J Photoenergy 2011:273683–27694
Preat J, Michaux C, Jacquemin D, Perpete EA (2009) J Phys Chem C 113:16821–16833
Preat J (2010) J Phys C 114:16716–16725
Pastore M, Mosconi E, De Angelis F, Grätzel M (2010) J Phys Chem C 114:7205–7212
Liu D, Fessenden RW, Hug GL, Kamat PV (1997) J Phys Chem B 101:2583–2590
Burfeindt B, Hannappel T, Storck W, Willig F (1996) J Phys Chem 100:16463–16465
Sayama K, Tsukagochi S, Hara K, Ohga Y, Shinpou A, Abe Y, Suga S, Arakawa H (2002) J Phys Chem B 106:1363–1371
Ning Z, Zhang Q, Wu W, Bo L, Tian H (2008) J Org Chem 73:3791–3797
Jamorski C, Lüthi HP (2002) J Chem Phys 117:4146–4156
Preat J, Jacquemin D, Wathelet V, André JM, Perpéte EA (2006) J Phys Chem A 110:8144–8150
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439–4449
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454–464
Turbomole: ab initio program, Ahlrichs R et al (1989) Chem Phys Lett 162:165–169
Frisch MJ, Trucks GW, Schlegel HB et al. (2003) Gaussian 09. Gaussian Inc, Pittsburgh
Klamt A, Schüürman G (1993) J Chem Soc Perkin Trans 2(5):799–805
Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlon CRA, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Keal TW, Sherwood P, Walsh A, Sokol AA (2013) Nat Mater 12:798–801
Chen J, Bai F-Q, Wang J, Hao L, Xie Z-F, Pan Q-J, Zhang H-X (2012) Dyes Pigments 94:459–468
Han L-H, Zhang C-R, Zhe JW, Jin N-Z, Shen Y-L, Wang W, Gong JJ, Chen Y-H, Liu Z-J (2013) Int J Mol Sci 14:20171–20188
Marcus RA (1993) Rev Mod Phys 65:599–610
Fan W, Tan D, Deng W-Q (2012) ChemPhysChem 13:2051–2060
Lee MJ, Balanay M, Kim D (2012) Theor Chem Acc 131:1269–1278
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1874
Torrent-Sucarrat M, De Proft F, Geerlings P, Ayers PW (2008) Chem Eur J 14:8652–8660
Acknowledgments
This work has been funded by Grants Conicyt-Aka-ERNC-001, Fondecyt 1140503 and 1150629, and Project RC120001 of the Iniciativa Científica Milenio (ICM) del Ministerio de Economía, Fomento y Turismo del Gobierno de Chile. N.I. wants to acknowledge the Fondecyt grant N° 11140770.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inostroza, N., Mendizabal, F., Arratia-Pérez, R. et al. Improvement of photovoltaic performance by substituent effect of donor and acceptor structure of TPA-based dye-sensitized solar cells. J Mol Model 22, 25 (2016). https://doi.org/10.1007/s00894-015-2893-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2893-9