Abstract
Background
Identification of predictors of clinical response to certolizumab-pegol (certolizumab) may aid the decision-making process for treating patients with rheumatoid arthritis (RA), spondyloarthritis (SpA), and psoriatic arthritis (PsA).
Objective
The aim of our study was to evaluate the effectiveness of certolizumab and identify any predictors of favorable outcome in patients with RA, PsA, or SpA.
Methods
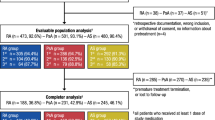
We studied 355 RA, SpA, and PsA patients starting treatment with certolizumab. Endpoints of the study were drug survival and identification of predictors of clinical outcome. Drug retention was analyzed via the Kaplan–Meier method, and hazard ratios (HRs) were estimated using Cox regression models.
Results
Of 355 certolizumab initiators, 178 had RA, 94 had PsA, and 83 had SpA. Biologic-naïve RA patients had significantly higher survival rates (73.3%) than switchers taking certolizumab as a second-line (49.0%) or third- or next-line biologic agent (51.2%; p = 0.0001). Instead, PsA and SpA patients showed similar drug retention rates regardless of the line of treatment. A significant clinical improvement from baseline was seen at 3 months for RA (28 joint-Disease Activity Score [DAS28]; p = 0.001), PsA (Disease Activity Index for PsA [DAPSA]; p = 0.001), and SpA (Bath Ankylosing Disease Index; p = 0.01). Biologic-naïve patients had the lowest HR (0.31; p = 0.001) of discontinuing certolizumab for RA, and the highest HR (7.94; p = 0.01) of achieving minimal disease activity (MDA) for PsA. For PsA, a predictor of late MDA was the achievement of low/remission DAPSA at 3 months, and 3-month low/remission DAS28 predicted late remission for RA.
Conclusions
Our study revealed that the best predictor of certolizumab effectiveness in unselected patients with RA, PsA, or SpA was a biologic-naïve status and achievement of an early response within 3 months.



Similar content being viewed by others
References
Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510.
Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–77.
van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–91.
Carmona L, Gómez-Reino JJ, BIOBADASER Group. Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther. 2006;8:R72.
Iannone F, Santo L, Anelli MG, Bucci R, Semeraro A, Quarta L, et al. Golimumab in real-life settings: 2 Years drug survival and predictors of clinical outcomes in rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum. 2017;47:108–14.
Thomas K, Flouri I, Repa A, Fragiadaki K, Sfikakis PP, Koutsianas C, et al. High 3-year golimumab survival in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis: real world data from 328 patients. Clin Exp Rheumatol. 2018;36:254–62.
Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47.
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73:48–55.
Smolen J, Landewé RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68:797–804.
Kumar N, Naz S, Quinn M, Ryan J, Kumke T, Sheeran T. Treatment of rheumatoid arthritis with certolizumab pegol: results from PROACTIVE, a non-interventional study in the UK and Ireland. Adv Ther. 2018;35:1426–37.
Iannone F, Carlino G, Marchesoni A, Sarzi-Puttini P, Gorla R, Lapadula G, GISEA (Gruppo Italiano di Studio sulle Early Arthritides). Early clinical response predicts low disease activity at one year in rheumatoid arthritis patients on treatment with certolizumab in real-life settings. An appraisal of the Italian registry GISEA. Jt Bone Spine. 2016;83:721–5.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73.
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83.
Iannone F, Lopalco G, Rigante D, Orlando I, Cantarini L, Lapadula G. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev. 2016;15:447–50.
Coates LC, Helliwell PS. Validation of minimal disease activity criteria for psoriatic arthritis using interventional trial data. Arthritis Care Res (Hoboken). 2010;62:965–9.
Berenbaum F, Pham T, Claudepierre P, de Chalus T, Joubert JM, Saadoun C, et al. Early non-response to certolizumab pegol in rheumatoid arthritis predicts treatment failure at one year. Data from a randomised phase III clinical trial. Jt Bone Spine. 2018;85:59–64.
van der Heijde D, Deodhar A, Fleischmann R, Mease PJ, Rudwaleit M, Nurminen T, et al. Early disease activity or clinical response as predictors of long-term outcomes with certolizumab pegol in axial spondyloarthritis or psoriatic arthritis. Arthritis Care Res (Hoboken). 2017;69:1030–9.
di Minno MN, Peluso R, Iervolino S, Lupoli R, Russolillo A, Scarpa R, et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res (Hoboken). 2013;65:141–7.
Vittecoq O, Richard L, Banse C, Lequerré T. The impact of smoking on rheumatoid arthritis outcomes. Jt Bone Spine. 2018;85:135–8.
Torrente-Segarra V, Bergstra SA, Salomon-Escoto K, Da Silva J, Veale DJ, Al-Emadi S, et al. Is current smoking status and its relationship to anti-cyclic citrullinated peptide antibodies a predictor of worse response to biological therapies in rheumatoid arthritis patients? Scand J Rheumatol. 2018;47(5):360–3.
Højgaard P, Glintborg B, Hetland ML, Hansen TH, Lage-Hansen PR, Petersen MH, et al. Association between tobacco smoking and response to tumour necrosis factor α inhibitor treatment in psoriatic arthritis: results from the DANBIO registry. Ann Rheum Dis. 2015;74:2130–6.
Acknowledgements
The authors are thankful to Massimiliano Dellisanti Fabiano Vilardi, Electronic Engineer, Ph.D., for his support in managing the BIOPURE registry and extracting the data for this study. The corresponding author declares that all authors approved all the submitted material and actively contributed to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Florenzo Iannone has received consultancy fees and/or speaker honoraria from Pfizer, AbbVie, MSD, BMS, Novartis, Lilly, and UCB outside this work. Angelo Semeraro has received speaker honoraria from Sanofi, Roche, AbbVie, BMS, MSD, and Novartis. Giorgio Carlino has received consultancy fees from Pfizer, Janssen, AbbVie, MSD, and BMS. Leonardo Santo has received consultancy fees and/or speaker honoraria from AbbVie, MSD, Novartis, and UCB outside this work. Romano Bucci has received consultancy fees and/or speaker honoraria from Pfizer, Sanofi, MSD, and BMS. Nicola Maruotti has received speaker honoraria from Pfizer outside this work. Carmelo Zuccaro has received consultancy fees and/or speaker honoraria from MSD, AbbVie, Novartis, Pfizer, and Janssen outside this work. Paola Chiara Falappone has received consultancy fees and/or speaker honoraria from Amgen, Abbott, MSD, and BMS outside this work. Francesco Paolo Cantatore has received consultancy fees and/or speaker honoraria from Pfizer and Roche outside this work. Giovanni Lapadula has received consultancy fees and/or speaker honoraria from Pfizer, AbbVie, MSD, BMS, and UCB outside this work. Laura Quarta, Daniela Mazzotta, Antonio Marsico, and Maurizio Muratore have no disclosures to declare.
Ethics approval
This study obtained the approval of the local ethics committees (Ethics Review Board of Policlinico of Bari (comitatoetico@policlinico.ba.it), protocol number 5277).
Informed consent
Patients gave their written informed consent to take part in this study and to use their data for publication, with explicit protection of identification. The study was conducted in compliance with the Declaration of Helsinki.
Funding
The BIOPURE registry has received unrestricted grants from Amgen, Roche, MSD, and UCB. UCB had no role in the study design or interpretation of the data. The submission of this article was unrelated to any approval from UCB.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version for publication. Dr. Iannone had full access to all of the data in this study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study conception and design: FI, AS, and GL. Acquisition of data: LS, RB, AS, LQ, NM, AM, GC, DM, CZ, PCF, FPC, MM, and GL. Analysis of data: FI and AS. Interpretation of data: All authors.
Rights and permissions
About this article
Cite this article
Iannone, F., Semeraro, A., Carlino, G. et al. Effectiveness of Certolizumab-Pegol in Rheumatoid Arthritis, Spondyloarthritis, and Psoriatic Arthritis Based on the BIOPURE Registry: Can Early Response Predict Late Outcomes?. Clin Drug Investig 39, 565–575 (2019). https://doi.org/10.1007/s40261-019-00782-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00782-9