Abstract
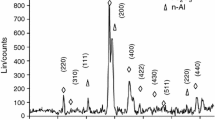
In this study, we use simultaneous differential scanning calorimetry and thermogravimetric analysis to analyze the solid-state reaction characteristics of Al/Fe2O3 nanothermites before n-Al melts. Different experimental setups for thermal analysis are compared, and the heat-release characteristics are studied on Al/Fe2O3 nanothermites in the form of powder and pellet, respectively. It is found that protection provided by mere argon flow is far from enough for thermal analysis of Al/Fe2O3 nanothermites. The experimental setup must be free of oxygen; otherwise, the determined exothermic peak is mostly the oxidation of n-Al by the residue oxygen molecules in the furnace body, due to the high reactivity and moderate accessibility of free oxygen molecules. Under proper experimental setup, it is found that besides the redox reaction between n-Al and n-Fe2O3, intermetallic reaction also occurs to form Fe4Al13. Moreover, the intermetallic reaction is more noticeable for nanothermite pellet than for nanothermite powder, and the eutectic reaction of Fe4Al13 and n-Al leads to melting peaks below 655 °C. The results indicate that instrument configuration and sample status affect the testing results to a large extent, and one should be prudent to derive reaction characteristics of nanothermites.








Similar content being viewed by others
References
Sundaram D, Yang V, Yetter RA (2017) Metal-based nanoenergetic materials: synthesis, properties, and applications. Prog Energy Combust Sci 61:293–365
Zhou X, Torabi M, Lu J, Shen R, Zhang K (2014) Nanostructured energetic composites: synthesis, ignition/combustion modeling, and applications. ACS Appl Mater Interfaces 6:3058–3074
Glavier L, Nicollet A, Jouot F et al (2017) Nanothermite/RDX-based miniature device for impact ignition of high explosives. Propellants Explos Pyrotech 42:308–317
Zhou X, Wang Y, Cheng Z, Ke X, Jiang W (2017) Facile preparation and energetic characteristics of core–shell Al/CuO metastable intermolecular composite thin film on a silicon substrate. Chem Eng J 328:585–590
Ru C, Wang F, Xu J et al (2017) Superior performance of a MEMS-based solid propellant microthruster (SPM) array with nanothermites. Microsyst Technol 23:3161–3174
Jang NS, Ha SH, Kim KH, Cho MH, Kim SH, Kim JM (2017) Low-power focused-laser-assisted remote ignition of nanoenergetic materials and application to a disposable membrane actuator. Combust Flame 182:58–63
Myagkov VG, Bykova LE, Zhigalov VS et al (2017) Thermite synthesis, structural and magnetic properties of Co–Al2O3 nanocomposite films. J Alloys Compd 724:820–826
Lafontaine E, Comet M (2016) Nanothermites. ISTE Ltd, London
Dreizin EL, Schoenitz M (2015) Correlating ignition mechanisms of aluminum-based reactive materials with thermoanalytical measurements. Prog Energy Combust Sci 50:81–105
Marin L, Gao Y, Vallet M et al (2017) Performance enhancement via incorporation of ZnO nanolayers in energetic Al/CuO multilayers. Langmuir 33:11086–11093
Ke X, Zhou X, Gao H et al (2018) Surface functionalized core/shell structured CuO/Al nanothermite with long-term storage stability and steady combustion performance. Mater Des 140:179–187
Kim JH, Cho MH, Kim KJ, Kim SH (2017) Laser ignition and controlled explosion of nanoenergetic materials: the role of multi-walled carbon nanotubes. Carbon 118:268–277
Kinsey AH, Slusarski K, Woll K, Gibbins D, Weihs TP (2016) Effect of dilution on reaction properties and bonds formed using mechanically processed dilute thermite foils. J Mater Sci 51:5738–5749. https://doi.org/10.1007/s10853-016-9876-9
Zhu Y, Zhou X, Xu J et al (2018) In situ preparation of explosive embedded CuO/Al/CL20 nanoenergetic composite with enhanced reactivity. Chem Eng J 354:885–895
Marin L, Warot-Fonrose B, Esteve A, Chabal YJ, Alfredo Rodriguez L, Rossi C (2016) Self-organized Al2Cu nanocrystals at the interface of aluminum-based reactive nanolaminates to lower reaction onset temperature. ACS Appl Mater Interfaces 8:13104–13113
Zhang T, Ma Z, Li G, Luo Y (2017) A new strategy for the fabrication of high performance reactive microspheres via energetic polyelectrolyte assembly. RSC Adv 7:904–913
Yan N, Qin L, Hao H, Hui L, Zhao F, Feng H (2017) Iron oxide/aluminum/graphene energetic nanocomposites synthesized by atomic layer deposition: enhanced energy release and reduced electrostatic ignition hazard. Appl Surf Sci 408:51–59
Williams RA, Schoenitz M, Ermoline A, Dreizin EL (2014) Low-temperature exothermic reactions in fully-dense Al/MoO3 nanocomposite powders. Thermochim Acta 594:1–10
Padhye R, Smith DK, Korzeniewski C, Pantoya ML (2017) Tailoring surface conditions for enhanced reactivity of aluminum powders with solid oxidizing agents. Appl Surf Sci 402:225–231
Xu D, Yang Y, Cheng H, Li YY, Zhang K (2012) Integration of nano-Al with Co3O4 nanorods to realize high-exothermic core–shell nanoenergetic materials on a silicon substrate. Combust Flame 159:2202–2209
Zhou X, Xu D, Yang G et al (2014) Highly exothermic and superhydrophobic Mg/fluorocarbon core/shell nanoenergetic arrays. ACS Appl Mater Interfaces 6:10497–10505
Zhao N, He C, Liu J et al (2014) Dependence of catalytic properties of Al/Fe2O3 thermites on morphology of Fe2O3 particles in combustion reactions. J Solid State Chem 219:67–73
Qin L, Yan N, Li J, Hao H, Zhao F, Feng H (2017) Enhanced energy performance from core–shell structured Al@Fe2O3 nanothermite fabricated by atomic layer deposition. RSC Adv 7:7188–7197
Sui H, Atashin S, Wen JZ (2016) Thermo-chemical and energetic properties of layered nano-thermite composites. Thermochim Acta 642:17–24
Zhang T, Ma Z, Li G, Wang Z, Zhao B, Luo Y (2016) Electrostatic interactions for directed assembly of high performance nanostructured energetic materials of Al/Fe2O3/multi-walled carbon nanotube (MWCNT). J Solid State Chem 237:394–403
Zhou X, Ke X, Jiang W (2016) Aluminum/copper oxide nanostructured energetic materials prepared by solution chemistry and electrophoretic deposition. RSC Adv 6:93863–93866
Ke X, Zhou X, Hao G, Xiao L, Liu J, Jiang W (2017) Rapid fabrication of superhydrophobic Al/Fe2O3 nanothermite film with excellent energy-release characteristics and long-term storage stability. Appl Surf Sci 407:137–144
Liu P, Li X, Cheng L, Zhu X, Li Y, Song D (2018) Preparation and characterization of n-Al/FeF3 nanothermite. Chem Eng J 331:850–855
Gedevanishvili S, Deevi SC (2002) Processing of iron aluminides by pressureless sintering through Fe + Al elemental route. Mater Sci Eng A 325:163–176
Li X, Scherf A, Heilmaier M, Stein F (2016) The Al-rich part of the Fe–Al phase diagram. J Phase Equilib Diffus 37:162–173
Sundman B, Ohnuma I, Dupin N, Kattner UR, Fries SG (2009) An assessment of the entire Al–Fe system including D03 ordering. Acta Mater 57:2896–2908
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51706105) and the Natural Science Foundation of Jiangsu Province (No. BK20170846).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Zhu, Y., Ke, X. et al. Exploring the solid-state interfacial reaction of Al/Fe2O3 nanothermites by thermal analysis. J Mater Sci 54, 4115–4123 (2019). https://doi.org/10.1007/s10853-018-3094-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-3094-6