Abstract
The aim of this work was to predict the permeability of two model drugs, sulfamerazine (SMR) and indomethacin (INM), and to determine the effect on their apparent permeabilities by complexation with cyclodextrins and/or meglumine or incorporation in microemulsions. Permeation experiments were performed using two-chamber diffusion cells with a new composition of bio-mimetic membrane composed of 80% of Lipoid® S100 and 20% of cholesterol in n-octanol 10% w/w solution, at 37 ± 0.5°C and 14,000 rpm. The predictive capacity of the permeability of passive diffusion absorbed compounds was evaluated using 20 drug standards and showed an exponential correlation between the apparent permeability coefficients (Papp) and the fraction absorbed percentages in humans (Fa%), with an R2 value of 0.67942 and a constant value of − 4.1 ± 0.8. SMR and INM were classified as Class II and I, respectively, according to the Biopharmaceutical Classification System. These drugs were complexed and incorporated in microemulsions. The Fa% from all the drug products was higher than 90%. SMR in the complexes and both drugs in microemulsions were classified as highly soluble. Thus, SMR and INM incorporated in these pharmaceutical products could be classified as Class I.




Similar content being viewed by others
REFERENCES
CDER/FDA. Guidance for Industry, Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system. Cent Drug Eval Res. 2017.
Corti G, Maestrelli F, Cirri M, Furlanetto S, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption I. Assessment of artificial membrane composition. Eur J Pharm Sci. 2006;27:346–53.
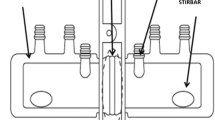
Giorno L, Mazzei R, Drioli E. Biological membranes and biomimetic artificial membranes. In: Drioli E, Giorno L, editors. Comprehensive membrane science and engineering. Oxford: Elsevier; 2010. p. 1–12.
Másson M, Loftsson T, Másson G, Stefánsson E. Cyclodextrins as permeation enhancers: some theoretical evaluations and in vitro testing. J Control Release. 1999;59:107–18.
Gantzsch SP, Kann B, Ofer-Glaessgen M, Loos P, Berchtold H, Balbach S, et al. Characterization and evaluation of a modified PVPA barrier in comparison to Caco-2 cell monolayers for combined dissolution and permeation testing. J Control Release. 2014;175:79–86.
Li G, Fan Y, Li X, Wang X, Li Y, Liu Y, et al. In vitro and in vivo evaluation of a simple microemulsion formulation for propofol. Int J Pharm. 2012;425:53–61.
Li H, Dong L, Liu Y, Wang G, Wang G, Qiao Y. Biopharmaceutics classification of puerarin and comparison of perfusion approaches in rats. Int J Pharm. 2014;466:133–8.
Silva AE, Barratt G, Chéron M, Egito EST. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int J Pharm. 2013;454:641–8.
Aloisio C, Gomes de Oliveira A, Longhi M. Characterization, inclusion mode, phase-solubility and in vitro release studies of inclusion binary complexes with cyclodextrins and meglumine using sulfamerazine as model drug. Drug Dev Ind Pharm. 2014;40:919–28.
Aloisio C, de Oliveira AG, Longhi M. Solubility and release modulation effect of sulfamerazine ternary complexes with cyclodextrins and meglumine. J Pharm Biomed Anal. 2014;100:64–73.
Aloisio C, Gomes de Oliveira A, Longhi M. G., de Oliveira A, Longhi M. Cyclodextrin and meglumine-based microemulsions as a poorly water-soluble drug delivery system. J Pharm Sci. 2015;In press:2703–11.
Aloisio C, Longhi MR, De Oliveira AG. Development and characterization of a biocompatible soybean oil-based microemulsion for the delivery of poorly water-soluble drugs. J Pharm Sci. 2015;10:3535–43.
Zhao G, Huang J, Xue K, Si L, Li G. Enhanced intestinal absorption of etoposide by self-microemulsifying drug delivery systems: roles of P-glycoprotein and cytochrome P450 3A inhibition. Eur J Pharm Sci. 2013;50:429–39.
Fang JY, Sung KC, Lin HH, Fang CL. Transdermal iontophoretic delivery of diclofenac sodium from various polymer formulations: in vitro and in vivo studies. Int J Pharm. 1999;178:83–92.
Fei Y, Kostewicz ES, Sheu M-T, Dressman JB. Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro–in silico–in vivo approach. Eur J Pharm Biopharm. 2013;85:1274–84.
Langguth P, Kubis A, Krumbiegel G, Lang W, Merkle HP, Wächter W, et al. Intestinal absorption of the quaternary trospium chloride: permeability-lowering factors and bioavailabilities for oral dosage forms. Eur J Pharm Biopharm. 1997;43:265–72.
Sjögren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernäs H, et al. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49:679–98.
Klimundová J, Šatinský D, Sklenářová H, Solich P, Satinský D, Sklenárová H. Automation of simultaneous release tests of two substances by sequential injection chromatography coupled with Franz cell. Talanta. 2006;69:730–5.
Sajeesh S, Bouchemal K, Marsaud V, Vauthier C, Sharma CP. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release. 2010;147:377–84.
Sugano K, Hamada H, Machida M, Ushio H, Saitoh K, Terada K. Optimized conditions of bio-mimetic artificial membrane permeation assay. Int J Pharm. 2001;228:181–8.
Samiei N, Mangas-Sanjuan V, González-Álvarez I, Foroutan M, Shafaati A, Zarghi A, et al. Ion-pair strategy for enabling amifostine oral absorption: rat in situ and in vivo experiments. Eur J Pharm Sci. 2013;49:499–504.
Sevin E, Dehouck L, Fabulas-da Costa A, Cecchelli R, Dehouck MP, Lundquist S, et al. Accelerated Caco-2 cell permeability model for drug discovery. J Pharmacol Toxicol. 2013;68:334–9.
Zhu M-L, Liang X-L, Zhao L-J, Liao Z-G, Zhao G-W, Cao Y-C, et al. Elucidation of the transport mechanism of baicalin and the influence of a Radix Angelicae Dahuricae extract on the absorption of baicalin in a Caco-2 cell monolayer model. J Ethnopharmacol. 2013;150:553–9.
Fujikawa M, Ano R, Nakao K, Akamatsu M. Relationships between structure and high-throughput screening permeability of diverse drugs with artificial membranes: application to prediction of Caco-2 cell permeability. Bioorg Med Chem. 2005;13:4721–32.
Sinkó B, Garrigues TM, Balogh GT, Nagy ZK, Tsinman O, Avdeef A, et al. Skin–PAMPA: a new method for fast prediction of skin penetration. Eur J Pharm Sci. 2012;45:698–707.
Sugano K, Nabuchi Y, Machida M, Aso Y. Prediction of human intestinal permeability using artificial membrane permeability. Int J Pharm. 2003;257:245–51.
Dezani AB, Pereira TM, Caffaro AM, Reis JM, Serra CH dos R, Helena C, et al. Determination of lamivudine and zidovudine permeability using a different ex vivo method in Franz cells. J Pharmacol Toxicol. 2013;67:194–202.
Velický M, Tam KY, Dryfe RAW. In situ artificial membrane permeation assay under hydrodynamic control: correlation between drug in vitro permeability and fraction absorbed in humans. Eur J Pharm Sci. 2011;44:299–309.
Levintova Y, Plakogiannis FM, Bellantone RA. An improved in vitro method for measuring skin permeability that controls excess hydration of skin using modified Franz diffusion cells. Int J Pharm. 2011;419:96–106.
Rauma M, Johanson G. Comparison of the thermogravimetric analysis (TGA) and Franz cell methods to assess dermal diffusion of volatile chemicals. Toxicol In Vitro. 2009;23:919–26.
Reis JM, Dezani AB, Pereira TM, Avdeef A, Serra CHR. Lamivudine permeability study: a comparison between PAMPA, ex vivo and in situ single-pass intestinal perfusion (SPIP) in rat jejunum. Eur J Pharm Sci. 2013;48:781–9.
Drugs.com. [Internet]. [Updated: 2018 Mar 1; Cited: 2018 April 5]. 2018. Available from: https://www.drugs.com/.
Amidon GL, Sinko PJ, Fleisher D. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res. 1988;5:651–4.
Lipka E, Amidon GL. Setting bioequivalence requirements for drug development based on preclinical data: optimizing oral drug delivery systems. J Control Release. 1999;62:41–9.
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186:119–25.
Paixão P, Gouveia LF, Morais JAG. Prediction of the human oral bioavailability by using in vitro and in silico drug related parameters in a physiologically based absorption model. Int J Pharm. 2012;429:84–98.
Delrivo A, Aloisio C, Longhi MR, Granero G. Artificial lipid membrane permeability method for predicting intestinal drug transport: probing the determining step in the oral absorption of sulfadiazine; influence of the formation of binary and ternary complexes with cyclodextrins. AAPS Pharm Sci Tech. 2018.
Chuasuwan B, Binjesoh V, Polli JE, Zhang H, Amidon GL, Junginger HE, et al. Biowaiver monographs for immediate release solid oral dosage forms: diclofenac sodium and diclofenac potassium. J Pharm Sci. 2009;98:1206–19.
Potthast H, Dressman JB, Junginger HE, Midha KK, Oeser H, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: ibuprofen. J Pharm Sci. 2005;94:2121–31.
Masaoka Y, Tanaka Y, Kataoka M, Sakuma S, Yamashita S. Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur J Pharm Sci. 2006;29:240–50.
Bhosale UV, Kusum Devi V, Jain N. Formulation and optimization of mucoadhesive nanodrug delivery system of acyclovir. J Young Pharm. 2011;3:275–83.
Fini A, Fazio G, Feroci G. Solubility and solubilization properties of non-steroidal anti-inflammatory drugs. Int J Pharm. 1995;126:95–102.
Gennaro AR. Volume 2. The Science and Practice of Pharmacy Remington farmacia, Joseph Price Remington. 20th ed. Buenos Aires: Ed. Médica Panamericana; 2003.
Thompson JE, Davidow LW. A practical guide to contemporary pharmacy. Lippincott Williams & Wilkins: Baltimore, Philadelphia; 2009.
Wishart D, Feunang Y, Guo A, Lo E, Marcu A, Grant J, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017; https://doi.org/10.1093/nar/gkx1037.2018.
Incecayir T, Tsume Y, Amidon G. Comparison of the permeability of metoprolol and labetalol in rat, mouse, and caco-2 cells: use as a reference standard for BCS classification. Mol Pharm. 2013:10958–66.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aloisio, C., Ponce-Ponte, M., Granero, G.E. et al. Effect of Complexes and Microemulsions on the Permeability of Drugs: Determination Using a New Biomimetic Artificial Membrane. AAPS PharmSciTech 19, 2629–2638 (2018). https://doi.org/10.1208/s12249-018-1096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1096-y