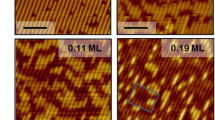
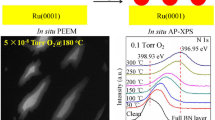
Abstract
Auger electron spectroscopy, high-resolution electron energy loss spectroscopy and temperature programmed desorption methods have been used in order to investigate the adsorption properties and reactions of acetaldehyde on gold decorated rhodium and BN/Rh(111) surfaces. Scanning tunneling microscopy and X-ray photoelectron spectroscopy measurements were carried out to characterize the gold nanoparticles on clean and hexagonal boron nitride (h-BN) covered Rh(111). The adsorption of acetaldehyde was not completely hindered by gold atoms; however, depending on the structure of the outermost bimetallic layer (surface alloy) the dissociation of the parent molecule was suppressed, namely the production of carbon monoxide was inhibited by the gold domains. Our measurements with acetaldehyde on Au/h-BN/Rh(111) confirmed the observation that the lack of suitable adsorption sites eliminates the formation of CO. Nevertheless, increased coverage of gold enhanced the amount of adsorbed aldehyde at low temperature. We may predict that the low reactivity of acetaldehyde on Au/h-BN/Rh(111) significantly determine the ethanol decomposition mechanism on this surface.







Similar content being viewed by others
References
Davis JL, Barteau MA (1987) Decarbonylation and decomposition pathways of alcohol’s on Pd(111). Surf Sci 187:387–406. https://doi.org/10.1016/S0039-6028(87)80064-X
Davis JL, Barteau MA (1988) The influence of oxygen on the selectivity of alcohol conversion on the Pd(111) surface. Surf Sci 197:123–152. https://doi.org/10.1016/0039-6028(88)90577-8
McCabe RW, Dimaggio CL, Madix RJ (1985) Adsorption and reactions of acetaldehyde on Pt(S)-[6(111) X (100)]. J Phys Chem 89:854–861. https://doi.org/10.1021/j100251a028
Mavrikakis M, Barteau MA (1998) Oxygenate reaction pathways on transition metal surfaces. J Mol Catal A Chem 131:135–147. https://doi.org/10.1016/S1381-1169(97)00261-6
Raskó J, Kiss J (2005) Adsorption and surface reactions of acetaldehyde on alumina-supported noble metal catalysts. Catal Lett 101:71–77. https://doi.org/10.1007/s10562-004-3752-y
Raskó J, Kecskés T, Kiss J (2005) FT-IR and mass spectrometric studies on the interaction of acetaldehyde with TiO2-supported noble metal catalysts. Appl Catal A Gen 287:244–251. https://doi.org/10.1016/j.apcata.2005.04.004
Roberts JM (1990) The atmospheric chemistry of organic nitrates. Atmos Environ 24A:243–287. https://doi.org/10.1016/0960-1686(90)90108-Y
Altshuller AP (1993) Production of aldehydes as primary emissions and from secondary atmospheric reactions of alkenes and alkanes during the night and early morning hours. Atmos Environ Part A Gen Top 27:21–32. https://doi.org/10.1016/0960-1686(93)90067-9
Yee A, Morrison SJ, Idriss H (2000) Reactions of ethanol over M/CeO2 catalysts. Evidence of carbon-carbon bond dissociation at low temperatures over Rh/CeO2. Catal Today 63:327–335. https://doi.org/10.1016/S0920-5861(00)00476-4
Mattos LV, Jacobs G, Davis BH, Noronha FB (2012) Production of hydrogen from ethanol: review of reaction mechanism and catalyst deactivation. Chem Rev 112:4094–4123
Ferencz Z, Erdohelyi A, Baán K et al (2014) Effects of support and Rh additive on co-based catalysts in the ethanol steam reforming reaction. ACS Catal 4:1205–1218. https://doi.org/10.1021/cs500045z
De Lima AFF, Colman RC, Zotin FMZ, Appel LG (2010) Acetaldehyde behavior over platinum based catalyst in hydrogen stream generated by ethanol reforming. Int J Hydrog Energy 35:13200–13205. https://doi.org/10.1016/j.ijhydene.2010.09.030
Varga E, Ferencz Z, Oszkó A et al (2015) Oxidation states of active catalytic centers in ethanol steam reforming reaction on ceria based Rh promoted Co catalysts: an XPS study. J Mol Catal A Chem 397:127–133. https://doi.org/10.1016/j.molcata.2014.11.010
Henderson MA, Zhou Y, White JM (1989) Polymerization and decomposition of acetaldehyde on Ru(001). J Am Chem Soc 111:1185–1193. https://doi.org/10.1021/ja00186a004
Davis JL, Barteau MA (1989) Polymerization and decarbonylation reactions of aldehydes on the Pd(111) surface. J Am Chem Soc 111:1782–1792. https://doi.org/10.1021/ja00187a035
Houtman CJ, Barteau MA (1991) Divergent pathways of acetaldehyde and ethanol decarbonylation on the Rh(111) surface. J Catal 130:528–546. https://doi.org/10.1016/0021-9517(91)90133-O
Kovács I, Farkas AP, Szitás Á et al (2017) Adsorption, polymerization and decomposition of acetaldehyde on clean and carbon-covered Rh(111) surfaces. Surf Sci. https://doi.org/10.1016/j.susc.2017.05.016
Karatok M, Vovk EI, Shah AA et al (2016) Erratum: acetaldehyde partial oxidation on the Au(111) model catalyst surface: C-C bond activation and formation of methyl acetate as an oxidative coupling product (Surface Science (2015) 641 (289-293) DOI: 10.1016/j.susc.2015.04.005). Surf Sci 649:152. https://doi.org/10.1016/j.susc.2016.01.011.
Meng Q, Shen Y, Xu J et al (2012) Mechanistic understanding of hydrogenation of acetaldehyde on Au(111): a DFT investigation. Surf Sci 606:1608–1617. https://doi.org/10.1016/j.susc.2012.06.014
Pan M, Flaherty DW, Mullins CB (2011) Low-temperature hydrogenation of acetaldehyde to ethanol on H-precovered Au(111). J Phys Chem Lett 2:1363–1367. https://doi.org/10.1021/jz200577n
Valden M (1998) Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281:1647–1650. https://doi.org/10.1126/science.281.5383.1647
Haruta M (1997) Size- and support-dependency in the catalysis of gold. Catal Today 36:153–166. https://doi.org/10.1016/S0920-5861(96)00208-8
Haruta M, Daté M (2001) Advances in the catalysis of Au nanoparticles. Appl Catal A Gen 222:427–437. https://doi.org/10.1016/S0926-860X(01)00847-X
Dumbuya K, Cabailh G, Lazzari R et al (2012) Evidence for an active oxygen species on Au/TiO2(110) model catalysts during investigation with in situ X-ray photoelectron spectroscopy. Catal Today 181:20–25. https://doi.org/10.1016/j.cattod.2011.09.035
Liu L, Zhou Z, Guo Q et al (2011) The 2-D growth of gold on single-layer graphene/Ru(0001): enhancement of CO adsorption. Surf Sci 605:L47–L50. https://doi.org/10.1016/j.susc.2011.04.040
Zhang Y, Zhang Y, Ma D et al (2013) Mn atomic layers under inert covers of graphene and hexagonal boron nitride prepared on Rh(111). Nano Res 6:887–896. https://doi.org/10.1007/s12274-013-0365-z
Gotterbarm K, Spath F, Bauer U et al (2015) Reactivity of graphene-supported Pt nanocluster arrays. ACS Catal 5:2397–2403. https://doi.org/10.1021/acscatal.5b00245
Corso M (2004) Boron nitride nanomesh. Science 303:217–220. https://doi.org/10.1126/science.1091979
Ng ML, Preobrajenski AB, Vinogradov AS, Mårtensson N (2008) Formation and temperature evolution of Au nanoparticles supported on the h-BN nanomesh. Surf Sci 602:1250–1255. https://doi.org/10.1016/j.susc.2008.01.028
Koch HP, Laskowski R, Blaha P, Schwarz K (2011) Adsorption of gold atoms on the h-BN/Rh(111) nanomesh. Phys Rev B 84:1–7. https://doi.org/10.1103/PhysRevB.84.245410
Koch HP, Laskowski R, Blaha P, Schwarz K (2012) Adsorption of small gold clusters on the h-BN/Rh(111) nanomesh. Phys Rev B 86:1–7. https://doi.org/10.1103/PhysRevB.86.155404
Patterson MC, Habenicht BF, Kurtz RL et al (2014) Formation and stability of dense arrays of Au nanoclusters on hexagonal boron nitride/Rh(111). Phys Rev B 89:1–10. https://doi.org/10.1103/PhysRevB.89.205423
Farkas AP, Török P, Solymosi F et al (2015) Investigation of the adsorption properties of borazine and characterisation of boron nitride on Rh(111) by electron spectroscopic methods. Appl Surf Sci 354:367–372. https://doi.org/10.1016/j.apsusc.2015.05.060
McKee WC, Patterson MC, Huang D et al (2016) CO adsorption on Au nanoparticles grown on hexagonal boron nitride/Rh(111). J Phys Chem C 120:10909–10918. https://doi.org/10.1021/acs.jpcc.6b01645
Gubó R, Vári G, Kiss J et al (2018) Tailoring the hexagonal boron nitride nanomesh on Rh(111) by gold. Phys Chem Chem Phys. https://doi.org/10.1039/C8CP00790J
Gazsi A, Koós A, Bánsági T, Solymosi F (2011) Adsorption and decomposition of ethanol on supported Au catalysts. Catal Today 160:70–78. https://doi.org/10.1016/j.cattod.2010.05.007
Óvári L, Berkó A, Vári G et al (2016) The growth and thermal properties of Au deposited on Rh(111): formation of an ordered surface alloy. Phys Chem Chem Phys 18:25230–25240. https://doi.org/10.1039/C6CP02128J
Furukawa J, Saegusa T, Fujii H et al (1960) Crystalline polyaldehydes. Die Makromol Chem 37:149–152. https://doi.org/10.1002/macp.1960.020370114
Zhao H, Kim J, Koel BE (2003) Adsorption and reaction of acetaldehyde on Pt(1 1 1) and Sn/Pt(1 1 1) surface alloys. Surf Sci 538:147–159. https://doi.org/10.1016/S0039-6028(03)00602-2
Guan Y, Hensen EJM (2013) Selective oxidation of ethanol to acetaldehyde by Au-Ir catalysts. J Catal 305:135–145. https://doi.org/10.1016/j.jcat.2013.04.023
Henderson MA, Radloff PL, White JM, Mims CA (1988) Surface chemistry of ketene on Ru(001). 1. Surface structures. J Phys Chem 92:11–41. https://doi.org/10.1021/j100325a025
Ćavar E, Westerström R, Mikkelsen A et al (2008) A single h-BN layer on Pt(1 1 1). Surf Sci 602:1722–1726. https://doi.org/10.1016/j.susc.2008.03.008
Frank M, Wolter K, Magg N et al (2001) Phonons of clean and metal-modified oxide films: an infrared and HREELS study. Surf Sci 492:270–284. https://doi.org/10.1016/S0039-6028(01)01475-3
Rokuta E, Hasegawa Y, Suzuki K et al (1997) Phonon dispersion of an epitaxial monolayer film of hexagonal boron nitride on Ni(111). Phys Rev Lett 79:4609–4612. https://doi.org/10.1103/PhysRevLett.79.4609
Berner S, Corso M, Widmer R et al (2007) Boron nitride nanomesh: functionality from a corrugated monolayer. Angew Chem Int Ed 46:5115–5119. https://doi.org/10.1002/anie.200700234
Bus E, Miller JT, Van Bokhoven JA (2005) Hydrogen chemisorption on Al2O3-supported gold catalysts. J Phys Chem B 109:14581–14587. https://doi.org/10.1021/jp051660z
Mavrikakis M, Stoltze P, Nørskov JK (2000) Making gold less noble. Catal Lett 64:101–106. https://doi.org/10.1023/A:1019028229377
Yoon B (2005) Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science 307:403–407. https://doi.org/10.1126/science.1104168
Chen M, Cai Y, Yan Z, Goodman DW (2006) On the origin of the unique properties of supported Au nanoparticles. J Am Chem Soc 128:6341–6346. https://doi.org/10.1021/ja0557536
Sterrer M, Yulikov M, Risse T et al (2006) When the reporter induces the effect: unusual IR spectra of CO on Au 1/MgO(001)/Mo(001). Angew Chem Int Ed 45:2633–2635. https://doi.org/10.1002/anie.200504473
Acknowledgements
Financial support of this work by the Hungarian Research Development and Innovation Office through grants GINOP-2.3.2-15-2016-00013 and NKFIH OTKA K120115 is gratefully acknowledged. The ELI-ALPS project (GINOP-2.3.6-15-2015-00001) is supported by the European Union and co-financed by the European Regional Development Fund. This research was also supported by the European Social Fund in the framework of TÁMOP-4.2.4.A/ 2–11/1-2012-0001 ‘National Excellence Program’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farkas, A.P., Szitás, Á., Vári, G. et al. Effect of Gold on the Adsorption Properties of Acetaldehyde on Clean and h-BN Covered Rh(111) Surface. Top Catal 61, 1247–1256 (2018). https://doi.org/10.1007/s11244-018-0979-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-0979-1