Abstract
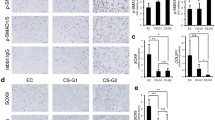
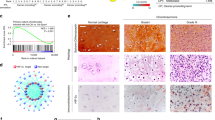
Approximately 75 % of extraskeletal myxoid chondrosarcoma tumors (EMC) harbor a t(9;22) chromosome translocation generating an EWS/NR4A3 fusion protein that is thought to be instrumental in the tumoral process. Current evidence suggests that one function of the fusion protein is to overexpress target genes. We have generated an in vitro human cellular model in which the fusion protein is expressed in mesenchymal bone marrow stem cells. We have performed microarray analyses of these cells and identified several genes overexpressed in the presence of EWS/NR4A3 which are also overexpressed in EMC tumors. These genes and their products represent potential therapeutic targets for EMC tumors.


Similar content being viewed by others
References
Labelle Y, Zucman J, Stenman G, Kindblom LG, Knight J, Turc-Carel C, Dockhorn-Dworniczak B, Mandahl N, Desmaze C, Peter M, et al. Oncogenic conversion of a novel orphan nuclear receptor by chromosome translocation. Hum Mol Genet. 1995;4:2219–26.
Brody RI, Ueda T, Hamelin A, Jhanwar SC, Bridge JA, Healey JH, Huvos AG, Gerald WL, Ladanyi M. Molecular analysis of the fusion of ews to an orphan nuclear receptor gene in extraskeletal myxoid chondrosarcoma. Am J Pathol. 1997;150:1049–58.
Clark J, Benjamin H, Gill S, Sidhar S, Goodwin G, Crew J, Gusterson BA, Shipley J, Cooper CS. Fusion of the ews gene to chn, a member of the steroid/thyroid receptor gene superfamily, in a human myxoid chondrosarcoma. Oncogene. 1996;12:229–35.
Attwooll C, Tariq M, Harris M, Coyne JD, Telford N, Varley JM. Identification of a novel fusion gene involving htafii68 and chn from a t(9;17)(q22;q11.2) translocation in an extraskeletal myxoid chondrosarcoma. Oncogene. 1999;18:7599–601.
Panagopoulos I, Mencinger M, Dietrich CU, Bjerkehagen B, Saeter G, Mertens F, Mandahl N, Heim S. Fusion of the rbp56 and chn genes in extraskeletal myxoid chondrosarcomas with translocation t(9;17)(q22;q11). Oncogene. 1999;18:7594–8.
Sjogren H, Meis-Kindblom J, Kindblom LG, Aman P, Stenman G. Fusion of the ews-related gene taf2n to tec in extraskeletal myxoid chondrosarcoma. Cancer Res. 1999;59:5064–7.
Sjogren H, Wedell B, Meis-Kindblom JM, Kindblom LG, Stenman G. Fusion of the nh2- terminal domain of the basic helix-loop-helix protein tcf12 to tec in extraskeletal myxoid chondrosarcoma with translocation t(9;15)(q22;q21). Cancer Res. 2000;60:6832–5.
Hisaoka M, Ishida T, Imamura T, Hashimoto H. Tfg is a novel fusion partner of nor1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2004;40:325–8.
Labelle Y, Bussieres J, Courjal F, Goldring MB. The ews/tec fusion protein encoded by the t(9;22) chromosomal translocation in human chondrosarcomas is a highly potent transcriptional activator. Oncogene. 1999;18:3303–8.
Filion C, Labelle Y. The oncogenic fusion protein ews/nor-1 induces transformation of cfk2 chondrogenic cells. Exp Cell Res. 2004;297:585–92.
Poulin H, Filion C, Ladanyi M, Labelle Y. Serum- and glucocorticoid-regulated kinase 1 (sgk1) induction by the ews/nor1(nr4a3) fusion protein. Biochem Biophys Res Commun. 2006;346:306–13.
Filion C, Motoi T, Olshen AB, Lae M, Emnett RJ, Gutmann DH, Perry A, Ladanyi M, Labelle Y. The ewsr1/nr4a3 fusion protein of extraskeletal myxoid chondrosarcoma activates the pparg nuclear receptor gene. J Pathol. 2009;217:83–93.
Sjogren H, Meis-Kindblom JM, Orndal C, Bergh P, Ptaszynski K, Aman P, Kindblom LG, Stenman G. Studies on the molecular pathogenesis of extraskeletal myxoid chondrosarcoma—cytogenetic, molecular genetic, and cdna microarray analyses. Am J Pathol. 2003;162:781–92.
Subramanian S, West RB, Marinelli RJ, Nielsen TO, Rubin BP, Goldblum JR, Patel RM, Zhu S, Montgomery K, Ng TL, Corless CL, Heinrich MC, van de Rijn M. The gene expression profile of extraskeletal myxoid chondrosarcoma. J Pathol. 2005;206:433–44.
Mihara K, Imai C, Coustan-Smith E, Dome JS, Dominici M, Vanin E, Campana D. Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. Br J Haematol. 2003;120:846–9.
Aigner T, Oliveira AM, Nascimento AG. Extraskeletal myxoid chondrosarcomas do not show a chondrocytic phenotype. Mod Pathol. 2004;17:214–21.
Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for ngfi-b by genetic selection in yeast. Science. 1991;252:1296–300.
Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for illumina microarray data. Nucleic Acids Res. 2008;36:e11.
Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42.
Park HJ, Kim SR, Kim MK, Choi KS, Jang HO, Yun I, Bae SK, Bae MK. Neuromedin b receptor antagonist suppresses tumor angiogenesis and tumor growth in vitro and in vivo. Cancer Lett. 2011;312:117–27.
Gregory CA, Singh H, Perry AS, Prockop DJ. The wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–78.
Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C. Derivation of sarcomas from mesenchymal stem cells via inactivation of the wnt pathway. J Clin Invest. 2007;117:3248–57.
Nagai MA, Fregnani JH, Netto MM, Brentani MM, Soares FA. Down-regulation of phlda1 gene expression is associated with breast cancer progression. Breast Cancer Res Treat. 2007;106:49–56.
Neef R, Kuske MA, Prols E, Johnson JP. Identification of the human phlda1/tdag51 gene: down-regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res. 2002;62:5920–9.
Chiu ST, Hsieh FJ, Chen SW, Chen CL, Shu HF, Li H. Clinicopathologic correlation of up-regulated genes identified using cDNA microarray and real-time reverse transcription-PCR in human colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:437–43.
Sakthianandeswaren A, Christie M, D’Andreti C, Tsui C, Jorissen RN, Li S, Fleming NI, Gibbs P, Lipton L, Malaterre J, Ramsay RG, Phesse TJ, Ernst M, Jeffery RE, Poulsom R, Leedham SJ, Segditsas S, Tomlinson IP, Bernhard OK, Simpson RJ, Walker F, Faux MC, Church N, Catimel B, Flanagan DJ, Vincan E, Sieber OM. PHLDA1 expression marks the putative epithelial stem cells and contributes to intestinal tumorigenesis. Cancer Res. 2011;71:3709–19.
Youssef J, Badr M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br J Pharmacol. 2011;164:68–82.
Naumann U, Huang H, Wolburg H, Wischhusen J, Weit S, Ohgaki H, Weller M. Pctaire3: a putative mediator of growth arrest and death induced by cts-1, a dominant-positive p53-derived synthetic tumor suppressor, in human malignant glioma cells. Cancer Gene Ther. 2006;13:469–78.
Valladares A, Hernandez NG, Gomez FS, Curiel-Quezada E, Madrigal-Bujaidar E, Vergara MD, Martinez MS, Arenas Aranda DJ. Genetic expression profiles and chromosomal alterations in sporadic breast cancer in Mexican women. Cancer Genet Cytogenet. 2006;170:147–51.
Kim JH, Zheng LT, Lee WH, Suk K. Pro-apoptotic role of integrin beta3 in glioma cells. J Neurochem. 2011;117:494–503.
Lei Y, Huang K, Gao C, Lau QC, Pan H, Xie K, Li J, Liu R, Zhang T, Xie N, Nai HS, Wu H, Dong Q, Zhao X, Nice EC, Huang C, Wei Y. Proteomics identification of ITGB3 as a key regulator in reactive oxygen species-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10:M110.005397.
Marin-Aguilera M, Mengual L, Burset M, Oliver A, Ars E, Ribal MJ, Colomer D, Mellado B, Villavicencio H, Algaba F, Alcaraz A. Molecular lymph node staging in bladder urothelial carcinoma: impact on survival. Eur Urol. 2008;54:1363–72.
Fung KM, Rorke LB, Giasson B, Lee VM, Trojanowski JQ. Expression of alpha-, beta-, and gamma-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106:167–75.
Kawashima M, Suzuki SO, Doh-ura K, Iwaki T. Alpha-synuclein is expressed in a variety of brain tumors showing neuronal differentiation. Acta Neuropathol. 2000;99:154–60.
Bruening W, Giasson BI, Klein-Szanto AJ, Lee VM, Trojanowski JQ, Godwin AK. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154–63.
Ye Q, Wang TF, Peng YF, Xie J, Feng B, Qiu MY, Li LH, Lu AG, Liu BY, Zheng MH. Expression of alpha-, beta- and gamma-synuclein in colorectal cancer, and potential clinical significance in progression of the disease. Oncol Rep. 2010;23:429–36.
Matsuo Y, Kamitani T. Parkinson’s disease-related protein, alpha-synuclein, in malignant melanoma. PloS One. 2010;5:e10481.
Xu Y, Josson S, Fang F, Oberley TD, St Clair DK, Wan XS, Sun Y, Bakthavatchalu V, Muthuswamy A, St Clair WH. Relb enhances prostate cancer growth: implications for the role of the nuclear factor-kappab alternative pathway in tumorigenicity. Cancer Res. 2009;69:3267–71.
dos Santos NR, Williame M, Gachet S, Cormier F, Janin A, Weih D, Weih F, Ghysdael J. Relb-dependent stromal cells promote t-cell leukemogenesis. PLoS One. 2008;3:e2555.
Jost PJ, Ruland J. Aberrant nf-kappab signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–7.
Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy Jr JD, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative nf-kappab pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30.
Demicco EG, Kavanagh KT, Romieu-Mourez R, Wang X, Shin SR, Landesman-Bollag E, Seldin DC, Sonenshein GE. RelB/p52 NF-kappaB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor ikappaB-alpha expression and promote carcinogenesis of the mammary gland. Mol Cell Biol. 2005;25:10136–47.
Sun P, Xia S, Lal B, Eberhart CG, Quinones-Hinojosa A, Maciaczyk J, Matsui W, Dimeco F, Piccirillo SM, Vescovi AL, Laterra J. DNER, an epigenetically modulated gene,regulates glioblastoma-derived neurosphere cell differentiation and tumor propagation. Stem Cells. 2009;27:1473–86.
Cheung AK, Ko JM, Lung HL, Chan KW, Stanbridge EJ, Zabarovsky E, Tokino T, Kashima L, Suzuki T, Kwong DL, Chua D, Tsao SW, Lung ML. Cysteine-rich intestinal protein 2 (CRIP2) acts as a repressor of nf-kappab-mediated proangiogenic cytokine transcription to suppress tumorigenesis and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:8390–5.
Acknowledgments
The authors thank Drs. Marc Ladanyi, Memorial Sloan-Kettering Cancer Center, and Bernard Têtu, Université Laval, for tumor samples. This work was supported by a grant from the Sarcoma Foundation of America (SFA10-11).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filion, C., Labelle, Y. Identification of genes regulated by the EWS/NR4A3 fusion protein in extraskeletal myxoid chondrosarcoma. Tumor Biol. 33, 1599–1605 (2012). https://doi.org/10.1007/s13277-012-0415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0415-2