Abstract
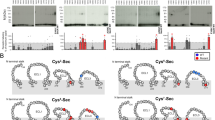
The interaction of insulin-degrading enzyme (IDE) with the main intracellular proteasome assemblies (i.e, 30S, 26S and 20S) was analyzed by enzymatic activity, mass spectrometry and native gel electrophoresis. IDE was mainly detected in association with assemblies with at least one free 20S end and biochemical investigations suggest that IDE competes with the 19S in vitro. IDE directly binds the 20S and affects its proteolytic activities in a bimodal fashion, very similar in human and yeast 20S, inhibiting at (IDE) ≤ 30 nM and activating at (IDE) ≥ 30 nM. Only an activating effect is observed in a yeast mutant locked in the “open” conformation (i.e., the α-3ΔN 20S), envisaging a possible role of IDE as modulator of the 20S “open”–”closed” allosteric equilibrium. Protein–protein docking in silico proposes that the interaction between IDE and the 20S could involve the C-term helix of the 20S α-3 subunit which regulates the gate opening of the 20S.







Similar content being viewed by others
References
Kirschner RJ, Goldberg AL (1983) A high molecular weight metalloendoprotease from the cytosol of mammalian cells. J Biol Chem 258:967–976
Kurochkin IV, Guarnera E, Berezovsky IN (2018) Insulin-degrading enzyme in the fight against Alzheimer’s Disease. Trends Pharmacol Sci 39:49–58
Yfanti C, Mengele K, Gkazepis A, Weirich G, Giersig C, Kuo WL, Tang WJ, Rosner M, Schmitt M (2008) Expression of metalloprotease insulin-degrading enzyme insulysin in normal and malignant humantissues. Int J Mol Med 4:421–431
Fernández-Gamba A, Leal MC, Morelli L, Castaño EM (2009) Insulin-degrading enzyme: structure–function relationship and its possible roles in health and disease. Curr Pharm Des 15:3644–3655
Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, Coletta M (2012) Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Asp Med 33:119–208
Song ES, Rodgers DW, Hersh LB (2018) Insulin-degrading enzyme is not secreted from cultured cells. Sci Rep 8:2335
Duckworth WC, Bennett RG, Hamel FG (1998) Insulin degradation: progress and potential. Endocr Rev 19:608–624
Kuroichkin IV (2001) Insulin-degrading enzyme: embarking on amyloid destruction. Trends Biochem Sci 26:421–425
Kurochkin IV, Goto S (1994) Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett 345(1):33–37
Ciaccio C, Tundo GR, Grasso G, Spoto G, Marasco D, Ruvo M, Gioia M, Rizzarelli E, Coletta M (2009) Somatostatin: a novel substrate and a modulator of insulin-degrading enzyme activity. J Mol Biol 385:1556–1567
Ralat LA, Kalas V, Zheng Z, Goldman RD, Sosnick TR, Tang WJ (2011) Ubiquitin is a novel substrate for human insulin-degrading enzyme. J Biol Chem 286:4670–4679
Grasso G, Salomone F, Tundo GR, Pappalardo G, Ciaccio C, Spoto G, Pietropaolo A, Coletta M, Rizzarelli E (2012) Metal ions affect insulin-degrading enzyme activity. J Inorg Biochem 117:351–358
Grasso G, Pietropaolo A, Spoto G, Pappalardo G, Tundo GR, Ciaccio C, Coletta M, Rizzarelli E (2011) Copper(I) and copper(II) inhibit Aβ peptides proteolysis by insulin-degrading enzyme differently: implications for metallostasis alteration in Alzheimer’s disease. Chemistry 17:2752–2762
Tundo GR, Ciaccio C, Sbardella D, Boraso MS, Viviani B, Coletta M, Marini S (2012) Somatostatin modulates insulin-degrading-enzyme metabolism: implications for the regulation of microglia activity in AD. PLoS One 7:e34376
Song ES, Jang H, Guo HF, Juliano MA, Juliano L, Morris AJ, Galperin E, Rodgers DW, Hersh LB (2017) Inositol phosphates and phosphoinositides activate insulin-degrading enzyme, while phosphoinositides also mediate binding to endosomes. Proc Natl Acad Sci USA 114:E2826–E2835
Song ES, Ozbil M, Zhang T, Sheetz M, Lee D, Tran D, Li S, Prabhakar R, Hersh LB, Rodgers DW (2015) An extended polyanion activation surface in insulin degrading enzyme. PLoS One 10:e0133114
Kochkina EG, Plesneva SA, Vasilev DS, Zhuravin IA, Turner AJ, Nalivaeva NN (2015) Effects of ageing and experimental diabetes on insulin-degrading enzyme expression in male rat tissues. Biogerontology 16:473–484
Tang WJ (2015) Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol Metab 27:24–34
Qiu W, Folstein M (2006) Insulin, insulin-degrading-enzyme and amyloid β-peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging 27:190–198
Deprez-Poulain R, Hennuyer N, Bosc D, Liang WG, Enée E, Marechal X, Charton J, Totobenazara J, Berte G, Jahklal J, Verdelet T, Dumont J, Dassonneville S, Woitrain E, Gauriot M, Paquet C, Duplan I, Hermant P, Cantrelle FX, Sevin E, Culot M, Landry V, Herledan A, Piveteau C, Lippens G, Leroux F, Tang WJ, van Endert P, Staels B, Deprez B (2015) Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice. Nat Commun 6:8250
Durham TB, Toth JL, Kimkovski VJ, Cao JX, Siesky AM, Alexander-Chacko J, Wu GY, Dixon JT, McGee JE, Wang J, Guo SY, Cavitt RN, Schindler J, Thibodeaux SJ, Calvert NA, Coghlan MJ, Sinderlar DK, Christe M, Kiselyov VV, Michael MD, Sloop KW (2015) Dual exosite-binding inhibitors of insulin-degrading enzyme challenge its role as the primary mediator of insulin clearance in vivo. J Biol Chem 290:20044–20059
Tundo GR, Sbardella D, Ciaccio C, Gioia M, Coletta A, Polticelli F, Di Pierro D, Milardi D, Van Endert P, Marini S, Coletta M (2017) Multiple functions of insulin-degrading enzyme: a metabolic crosslight? Crit Rev Biochem Mol Biol 21:1–29
Parmentier N, Stroobant V, Colau D, de Diesbach P, Morel S, Chapiro J, van Endert P, Van den Heynde B (2010) Production of an antigenic peptide by insulin-degrading enzyme. Nat Immunol 11:449–454
Radulescu RT, Duckworth WC, Levy JL, Fawcett J (2010) Retinoblastoma protein co-purifies with proteasomal insulin-degrading enzyme: implications for cell proliferation control. Biochem Biophys Res Commun 395:196–199
Tundo GR, Sbardella D, Ciaccio C, Bianculli A, Orlandi A, Desimio MG, Arcuri G, Coletta M, Marini S (2013) Insulin-degrading enzyme (IDE): a novel heat shock-like protein. J Biol Chem 288:2281–2289
Sbardella D, Tundo GR, Sciandra F, Bozzi M, Gioia M, Ciaccio C, Tarantino U, Brancaccio A, Coletta M, Marini S (2015) 26S proteasome activity is affected by fluctuations in insulin-degrading enzyme distribution. PLoS One 10:e0132455
Tundo GR, Sbardella D, De Pascalis SA, Ciaccio C, Coletta M, Fanizzi FP, Marini S (2015) Novel platinum(II) compounds modulate insulin-degrading enzyme activity and induce cell death in neuroblastoma cells. J Biol Inorg Chem 20:101–108
Hamel FG, Bennett RG, Duckworth WC (1998) Regulation of multicatalytic enzyme activity by insulin and the insulin-degrading enzyme. Endocrinology 139:4061–4066
Bennett RG, Hamel FG, Duckworth WC (2000) Insulin inhibits the ubiquitin-dependent degrading activity of the 26S proteasome. Endocrinology 141:2508–2517
Bennett RG, Fawcett J, Kruer MC, Duckworth WC, Hamel FG (2003) Insulin inhibition of the proteasome is dependent on degradation of insulin-degrading enzyme. J Endocrinol 177:399–405
Beuzelin C, Evnouchidou I, Rigolet P, Cauvet-Burgevin A, Girard PM, Dardalhon D, Culina S, Gdoura A, van Endert P, Francesconi S (2013) Deletion of the fission yeast homologue of human insulinase reveals a TORC1-dependent pathway mediating resistance to proteotoxic stress. PLoS One 8:e67705
Grasso G, Lanza V, Malgieri G, Fattorusso R, Pietropaolo A, Rizzarelli E, Milardi D (2015) The insulin degrading enzyme activates ubiquitin and promotes the formation of K48 and K63 diubiquitin. Chem Commun (Camb) 51:15724–15727
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Glickman MH, Ciechanover A (2002) The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Tomko RJ Jr, Hochstrasser M (2013) Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem 82:415–445
Schmidt M, Finley D (2014) Regulation of proteasome activity in health and disease. Biochem Biophys Acta 1843:13–25
Köhler A, Cascio P, Leggett DS, Woo KM, Goldberg AL, Finley D (2011) The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol Cell 7:1143–1152
Groll M, Nazif T, Huber R, Bogyo M (2002) Probing structural determinants distal to the site of hydrolysis that control substrate specificity of the 20S proteasome. Chem Biol 9:655–662
Rabl J, Smith DM, Yu Y, Chang SC, Goldberg AL, Cheng Y (2008) Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell 30:360–368
Murata S, Yashiroda H, Tanaka K (2009) Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol 10:104–115
Finley D (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78:477–513
Santoro AM, Cunsolo A, D’Urso A, Sbardella D, Tundo GR, Ciaccio C, Coletta M, Diana D, Fattorusso R, Persico M, Di Dato A, Fattorusso C, Milardi D, Purrello R (2016) Cationic porphyrins are tunable gatekeepers of the 20S proteasome. Chem Sci 7:1286–1297
Schweitzer A, Aufderheide A, Rudack T, Beck F, Pfeifer G, Plitzko JM, Sakata E, Schulten K, Förster F, Baumeister W (2016) Structure of the human 26S proteasome at a resolution of 3.9 Å. Proc Natl Acad Sci USA 113:7816–7821
Sledź P, Förster F, Baumeister W (2013) Allosteric effects in the regulation of 26S proteasome activities. J Mol Biol 425:1415–1423
Lasker K, Forster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P, Beck F, Aebersold R, Sali A, Baumeister W (2012) Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci USA 109:1380–1387
da Fonseca PCA, He J, Morris EP (2012) Molecular model of the human 26S proteasome. Mol Cell 46:54–66
Groll M, Bajorek M, Kohler A, Moroder L, Rubin DM, Huber R, Glickman MH, Finley D (2000) A gated channel into the proteasome core particle. Nat Struct Biol 7:1062–1067
Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A (2012) Complete subunit architecture of the proteasome regulatory particle. Nature 482:186–191
Leggett DS, Hanna J, Borodovsky A, Crosas B, Schmidt M, Baker RT, Walz T, Ploegh H, Finley D (2002) Multiple associated proteins regulate proteasome structure and function. Mol Cell 10:495–507
Ustrell V, Hoffman L, Pratt G, Rechsteiner M (2002) PA200, a nuclear proteasome activator involved in DNA repair. EMBO J 21:3516–3525
Glickman MH, Raveh D (2005) Proteasome plasticity. FEBS Lett 579:3214–3223
Stadtmueller BM, Hill CP (2011) Proteasome activators. Mol Cell 41:8–19
Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T (1998) Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J 335:637–642
Davies KJ (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83:301–310
Grune T, Catagol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies JK (2011) HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med 51:1355–1364
Raynes R, Pomatto LC, Davies KJ (2016) Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol Asp Med 50:41–55
Livnat-Levanon N, Kevei É, Kleifeld O, Krutauz D, Segref A, Rinaldi T, Erpapazoglou Z, Cohen M, Reis N, Hoppe T, Glickman MH (2014) Reversible 26S proteasome disassembly upon mitochondrial stress. Cell Rep 7:1371–1380
Elsasser S, Schmidt M, Finley D (2005) Characterization of the proteasome using native gel electrophoresis. Methods Enzymol 398:353–363
Ritchie DW, Venkatraman V (2010) Ultra-fast FFT protein docking on graphics processors. Bioinformatics 26:2398–2405
Shen Y, Joachimiak A, Rosner MR, Tang WJ (2006) Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature 443:870–874
Šali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Bousquet-Dubouch MP, Baudelet E, Guerin F, Matondo M, Uttenweller-Joseph S, Burlet-Schiltz O, Monsarrat B (2009) Affinity purification strategy to capture human endogenous proteasome complexes diversity and to identify proteasome-interacting proteins. Mol Cell Proteom 8:1150–1164
Fabre B, Lambour T, Garrigues L, Ducoux-Petit M, Amalric F, Monsarrat B, Burlet-Schiltz O, Bousquet-Dubouch MP (2014) Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J Proteome Res 13:3027–3037
Neelam S, Kakhniashvili DG, Wilkens S, Levene SD, Goodman SR (2011) Functional 20S proteasomes in mature human red blood cells. Exp Biol Med 236:580–591
de Tullio MB, Castelletto V, Hamley IW, Martino Adami PV, Morelli L, Castaño EM (2013) Proteolytically inactive insulin-degrading enzyme inhibits amyloid formation yielding non-neurotoxic aβ peptide aggregates. PLoS One 8:e59113
Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P (2015) Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep 5:12531
Hersh LB (2006) The insulysin (insulin degrading enzyme) enigma. Cell Mol Life Sci 63:2432–2434
Acknowledgements
The authors thank Prof. Michael Glickman and Prof. Peter van Endert, for several fruitful discussions. We are grateful to Prof. Michael Groll for the generous gift of wt y20S and α-3ΔN mutant proteasome. The financial support from the Italian Ministry of University and Research (MiUR PRIN20157WZM8 to G.G. and S.M.) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All experiments have been carried out according to the current laws of the country where they have been performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sbardella, D., Tundo, G.R., Coletta, A. et al. The insulin-degrading enzyme is an allosteric modulator of the 20S proteasome and a potential competitor of the 19S. Cell. Mol. Life Sci. 75, 3441–3456 (2018). https://doi.org/10.1007/s00018-018-2807-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2807-y